Deposition Date
2016-04-16
Release Date
2016-05-11
Last Version Date
2024-05-08
Entry Detail
PDB ID:
5G2X
Keywords:
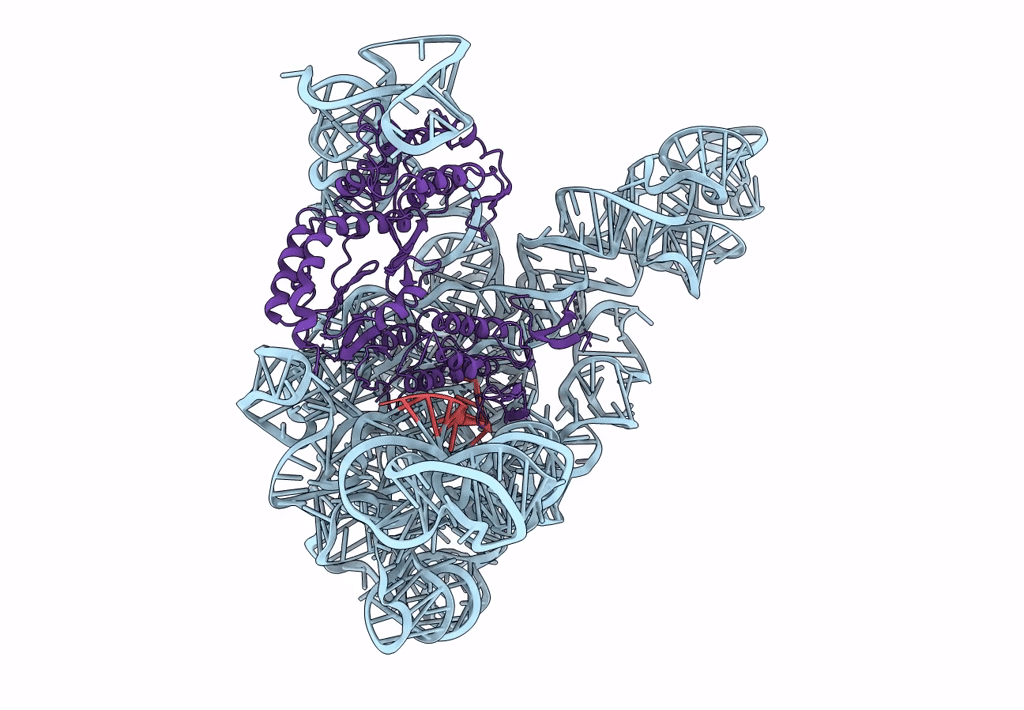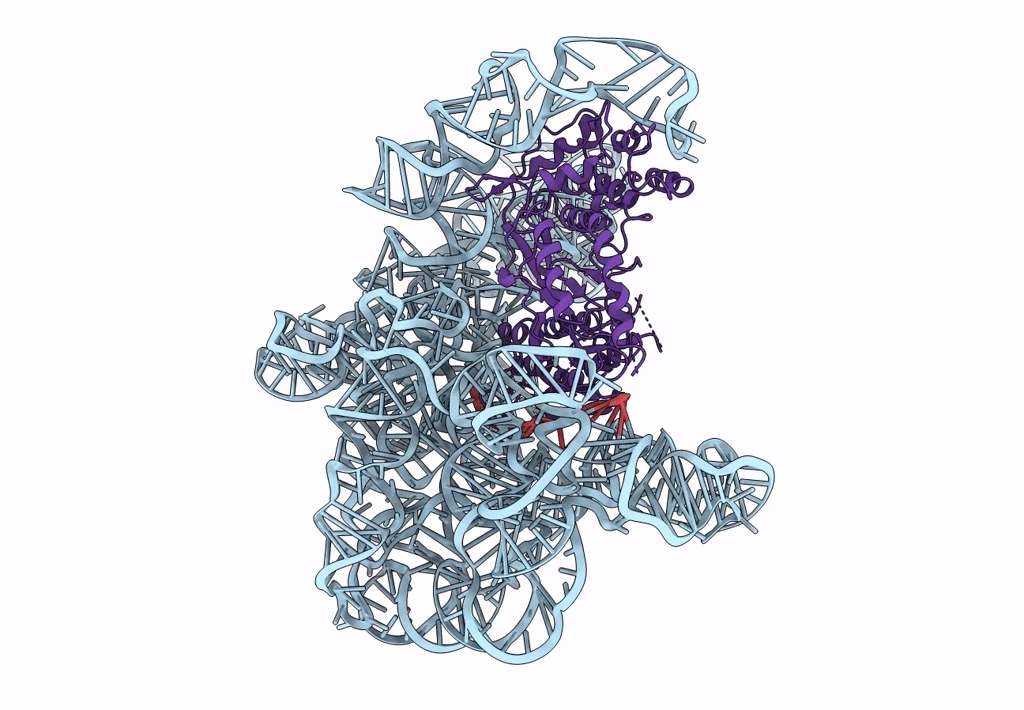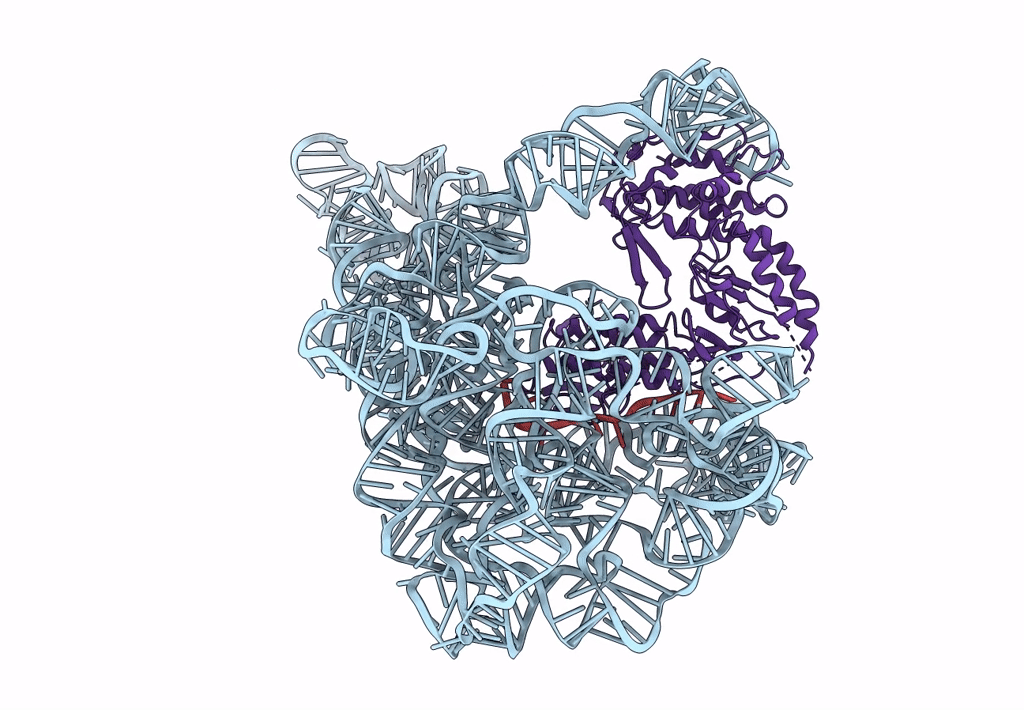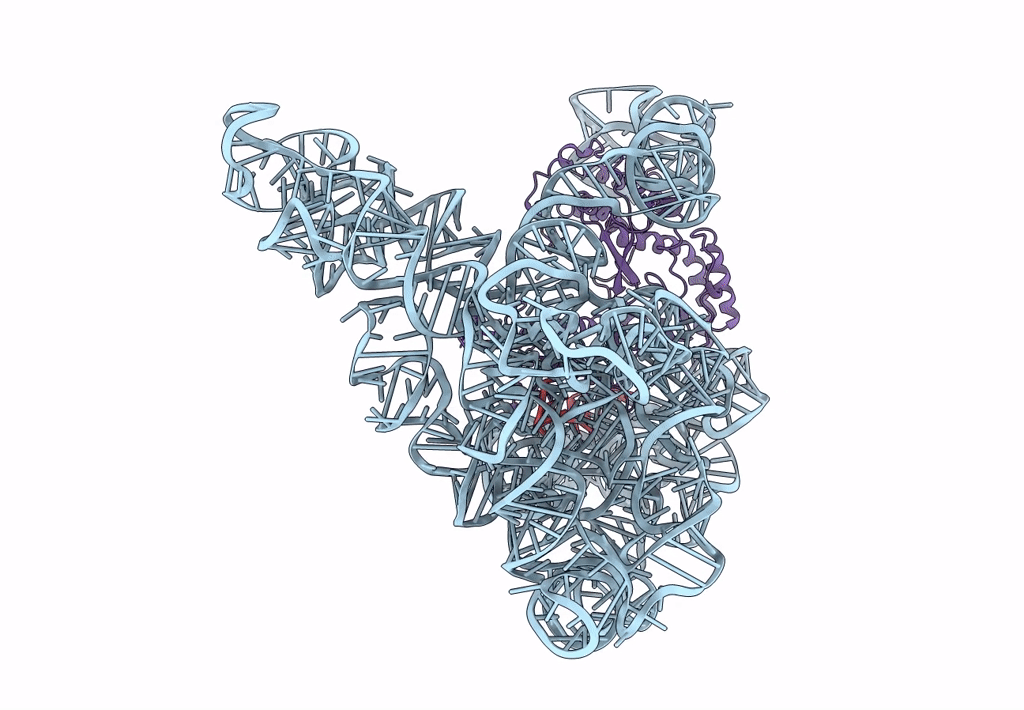
Title:
Structure a of Group II Intron Complexed with its Reverse Transcriptase
Biological Source:
Source Organism(s):
LACTOCOCCUS LACTIS (Taxon ID: 1358)
LACTOCOCCUS LACTIS SUBSP. CREMORIS (Taxon ID: 1359)
LACTOCOCCUS LACTIS SUBSP. CREMORIS (Taxon ID: 1359)
Expression System(s):
Method Details:
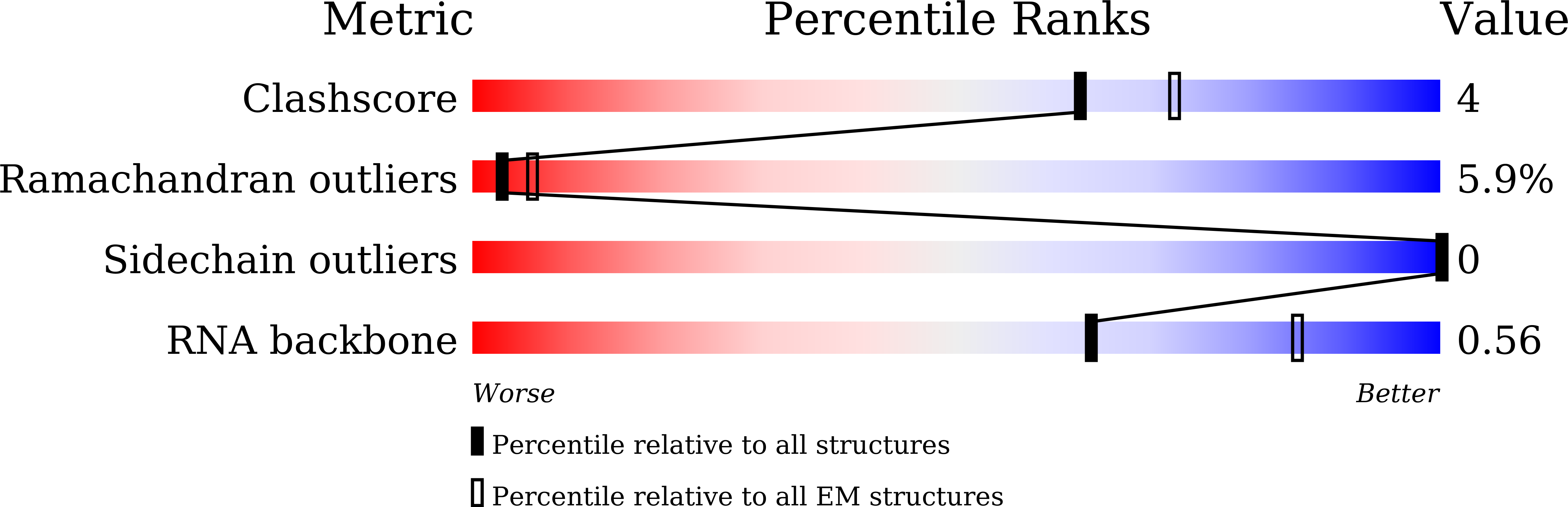
Experimental Method:
Resolution:
3.80 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE