Deposition Date
2016-03-08
Release Date
2016-04-27
Last Version Date
2024-11-13
Entry Detail
PDB ID:
5FYK
Keywords:
Title:
Crystal Structure at 3.7 A Resolution of Fully Glycosylated HIV-1 Clade B JR-FL SOSIP.664 Prefusion Env Trimer in Complex with Broadly Neutralizing Antibodies PGT122, 35O22 and VRC01
Biological Source:
Source Organism(s):
HUMAN IMMUNODEFICIENCY VIRUS 1 (Taxon ID: 11676)
HOMO SAPIENS (Taxon ID: 9606)
HOMO SAPIENS (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.11 Å
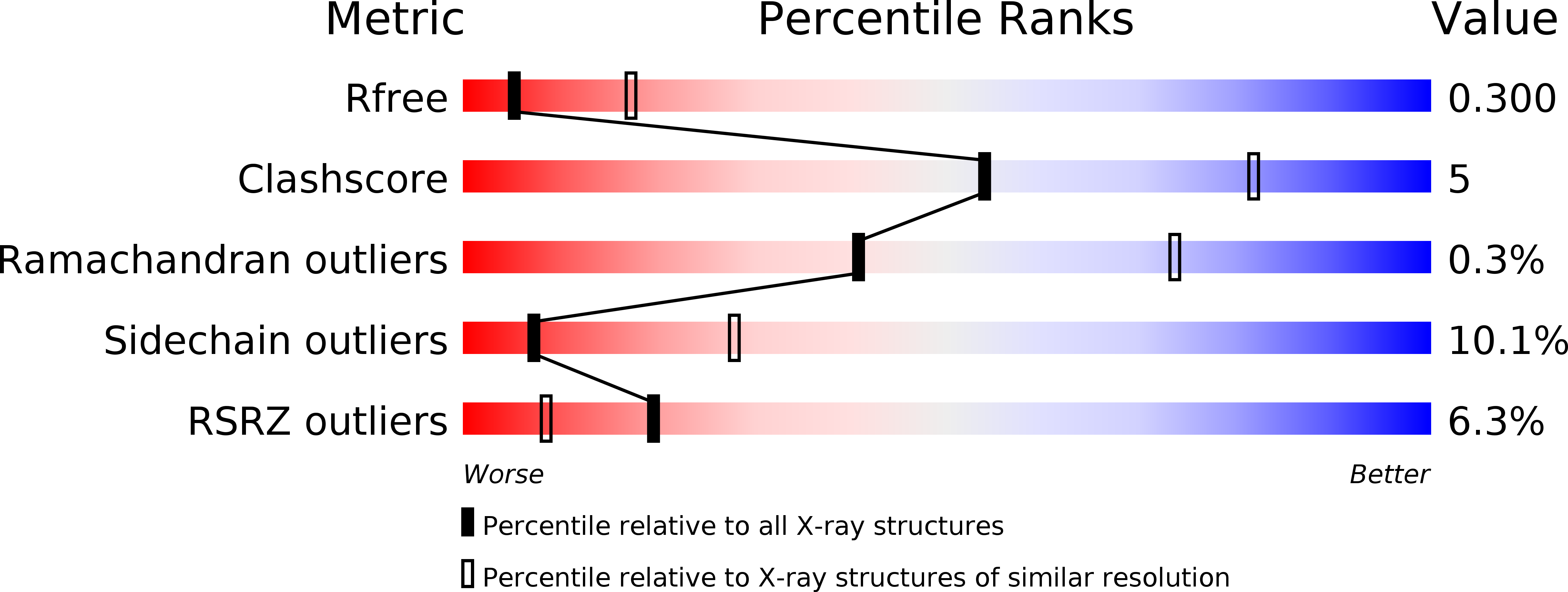
R-Value Free:
0.30
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
P 63