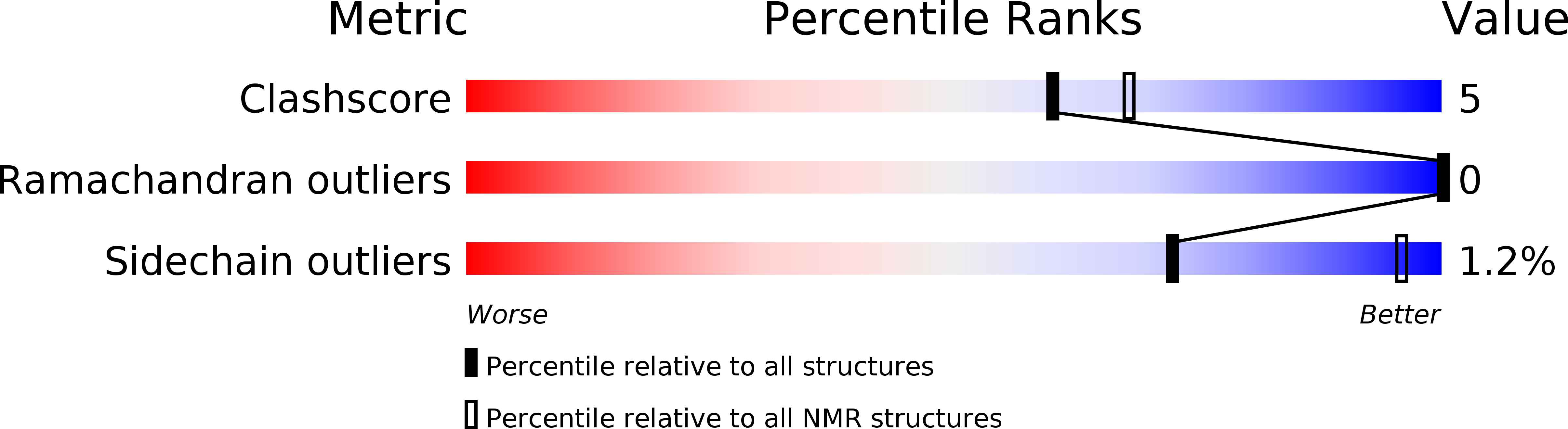Deposition Date
2015-12-17
Release Date
2016-05-04
Last Version Date
2024-06-19
Entry Detail
PDB ID:
5FRG
Keywords:
Title:
The NMR Structure of the Cdc42-interacting region of TOCA1
Biological Source:
Source Organism(s):
XENOPUS (SILURANA) TROPICALIS (Taxon ID: 8364)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
35
Selection Criteria:
LOWEST ENERGY