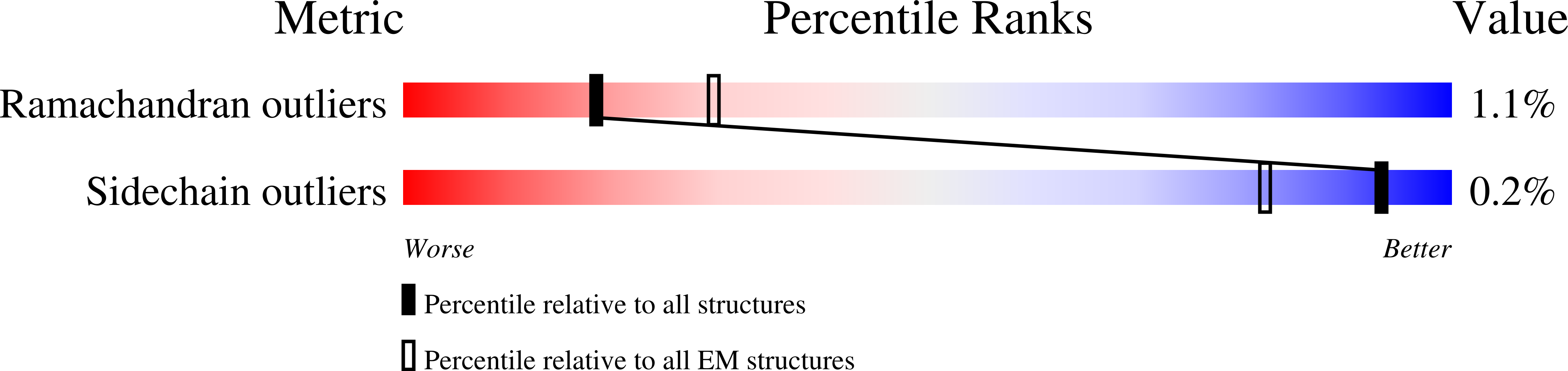Deposition Date
2015-11-04
Release Date
2016-03-02
Last Version Date
2024-11-13
Entry Detail
PDB ID:
5FMG
Keywords:
Title:
Structure and function based design of Plasmodium-selective proteasome inhibitors
Biological Source:
Source Organism(s):
PLASMODIUM FALCIPARUM (Taxon ID: 5833)
Method Details:
Experimental Method:
Resolution:
3.60 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE