Deposition Date
2015-10-01
Release Date
2016-01-20
Last Version Date
2024-01-10
Entry Detail
PDB ID:
5FIP
Keywords:
Title:
Discovery and characterization of a novel thermostable and highly halotolerant GH5 cellulase from an Icelandic hot spring isolate
Biological Source:
Source Organism(s):
UNIDENTIFIED (Taxon ID: 32644)
Method Details:
Experimental Method:
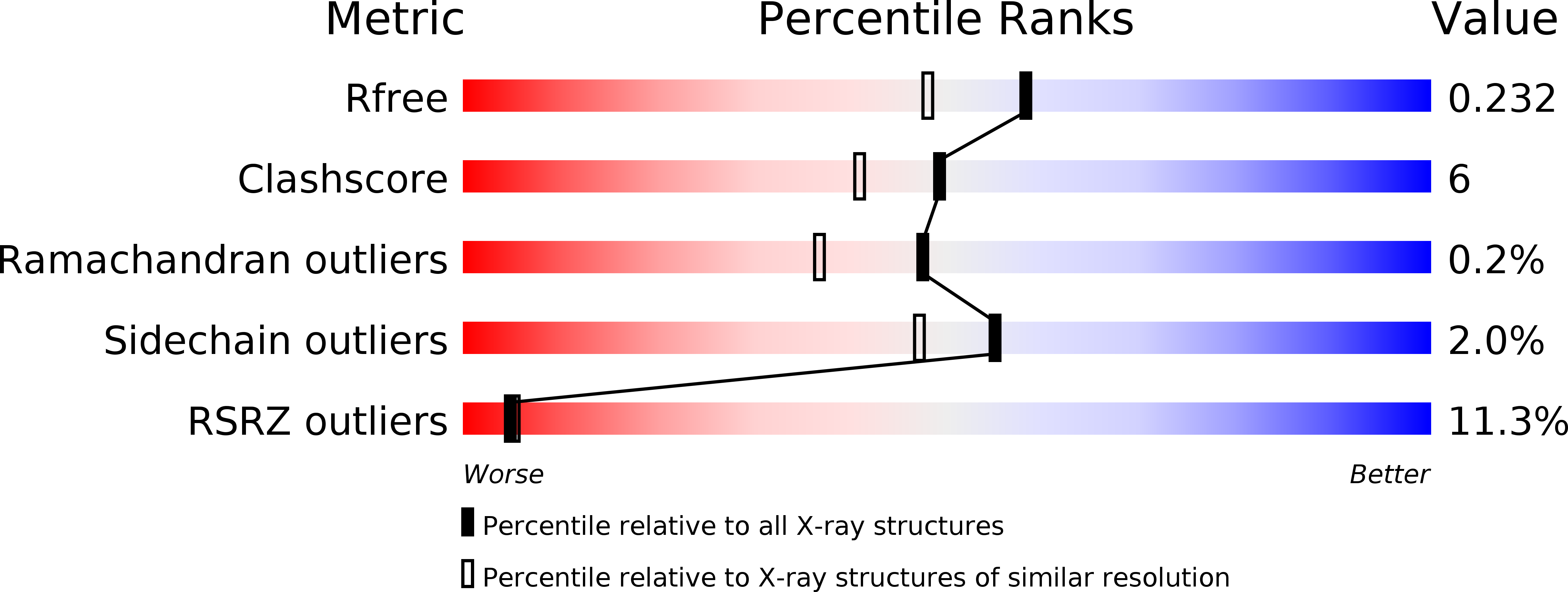
Resolution:
1.88 Å
R-Value Free:
0.23
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 1 2 1