Deposition Date
2015-12-16
Release Date
2016-05-25
Last Version Date
2024-11-06
Entry Detail
PDB ID:
5FDJ
Keywords:
Title:
Hen egg lysozyme at room temperature solved from datasets acquired by ultrasonic acoustic levitation method and processed by CrystFEL
Biological Source:
Source Organism(s):
Gallus gallus (Taxon ID: 9031)
Method Details:
Experimental Method:
Resolution:
1.80 Å
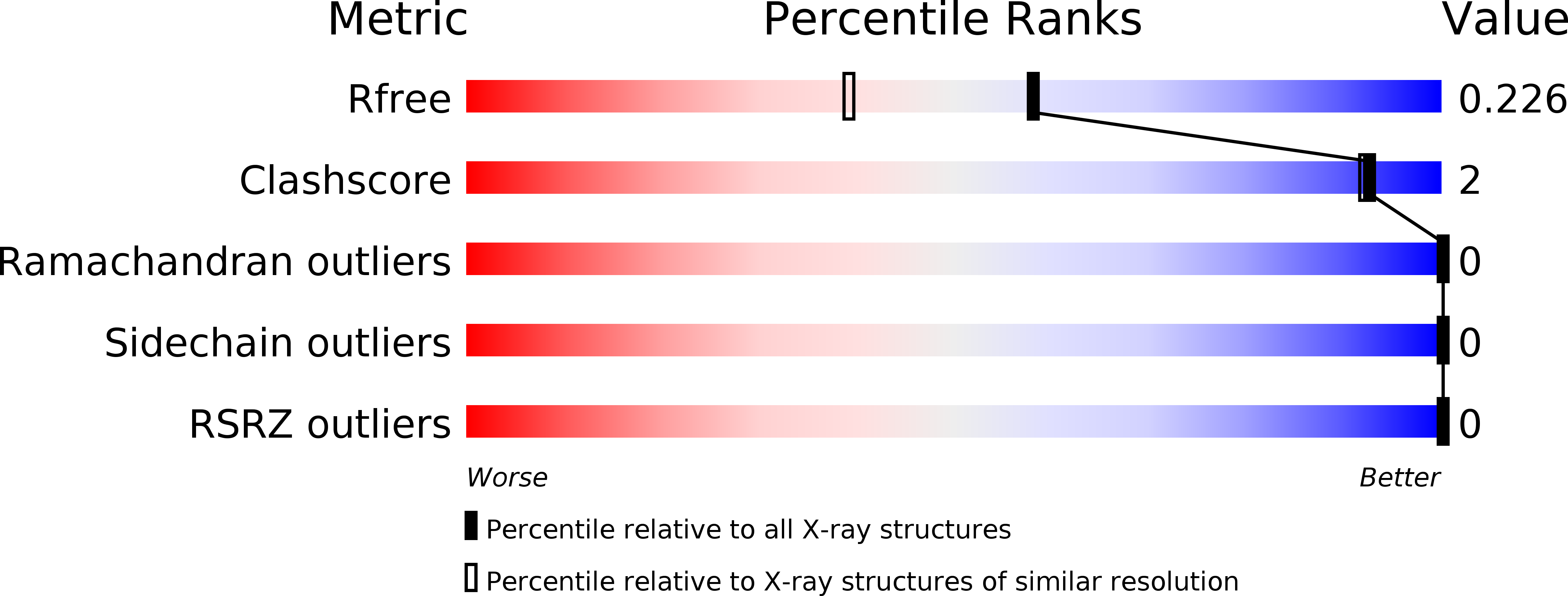
R-Value Free:
0.21
R-Value Work:
0.17
R-Value Observed:
0.18
Space Group:
P 43 21 2