Deposition Date
2015-11-26
Release Date
2016-03-09
Last Version Date
2024-11-13
Entry Detail
PDB ID:
5EZV
Keywords:
Title:
X-ray crystal structure of AMP-activated protein kinase alpha-2/alpha-1 RIM chimaera (alpha-2(1-347)/alpha-1(349-401)/alpha-2(397-end) beta-1 gamma-1) co-crystallized with C2 (5-(5-hydroxyl-isoxazol-3-yl)-furan-2-phosphonic acid)
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.99 Å
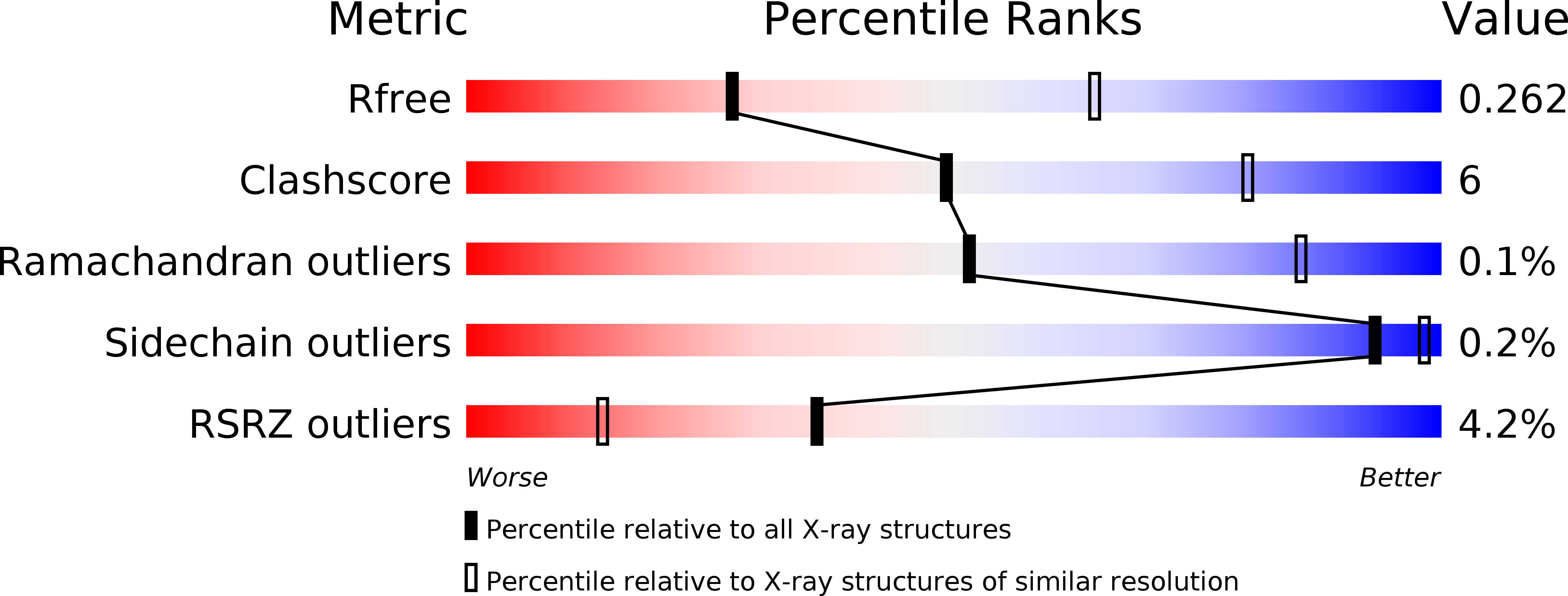
R-Value Free:
0.24
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 1 21 1