Deposition Date
2015-11-26
Release Date
2016-11-30
Last Version Date
2024-05-08
Entry Detail
PDB ID:
5EZU
Keywords:
Title:
Crystal structure of the N-terminal domain of vaccinia virus immunomodulator A46 in complex with myristic acid.
Biological Source:
Source Organism:
Vaccinia virus (strain Western Reserve) (Taxon ID: 10254)
Host Organism:
Method Details:
Experimental Method:
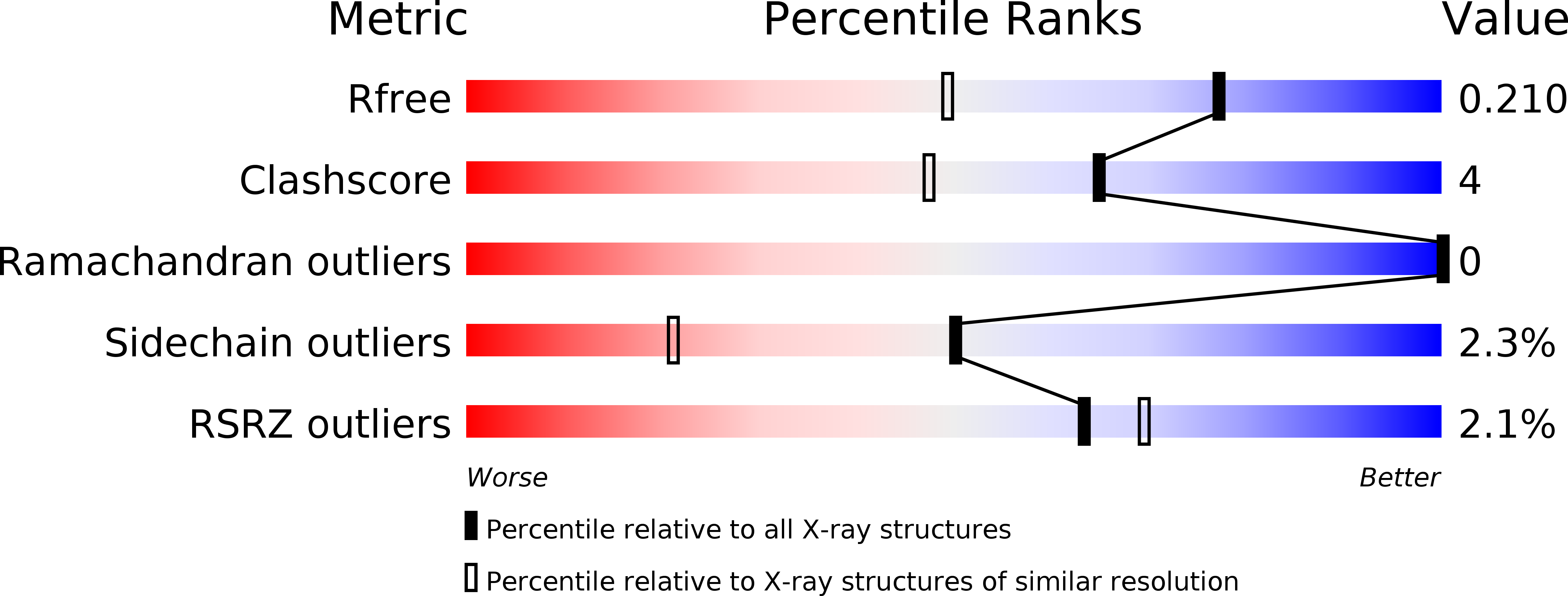
Resolution:
1.55 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
C 1 2 1