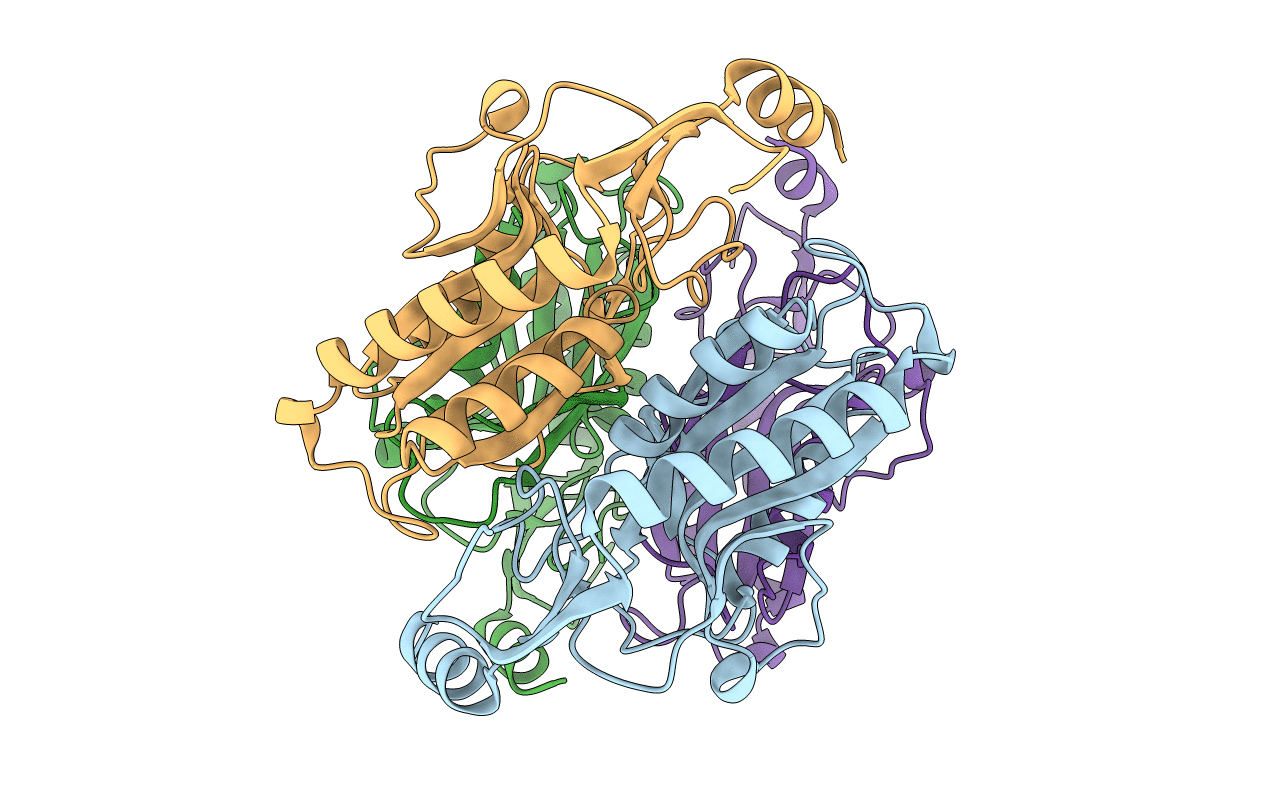
Deposition Date
2015-11-18
Release Date
2016-03-30
Last Version Date
2023-09-27
Entry Detail
PDB ID:
5EUC
Keywords:
Title:
The role of the C-terminal region on the oligomeric state and enzymatic activity of Trypanosoma cruzi hypoxanthine phosphoribosyl transferase
Biological Source:
Source Organism(s):
Trypanosoma cruzi (strain CL Brener) (Taxon ID: 353153)
Expression System(s):
Method Details:
Experimental Method:
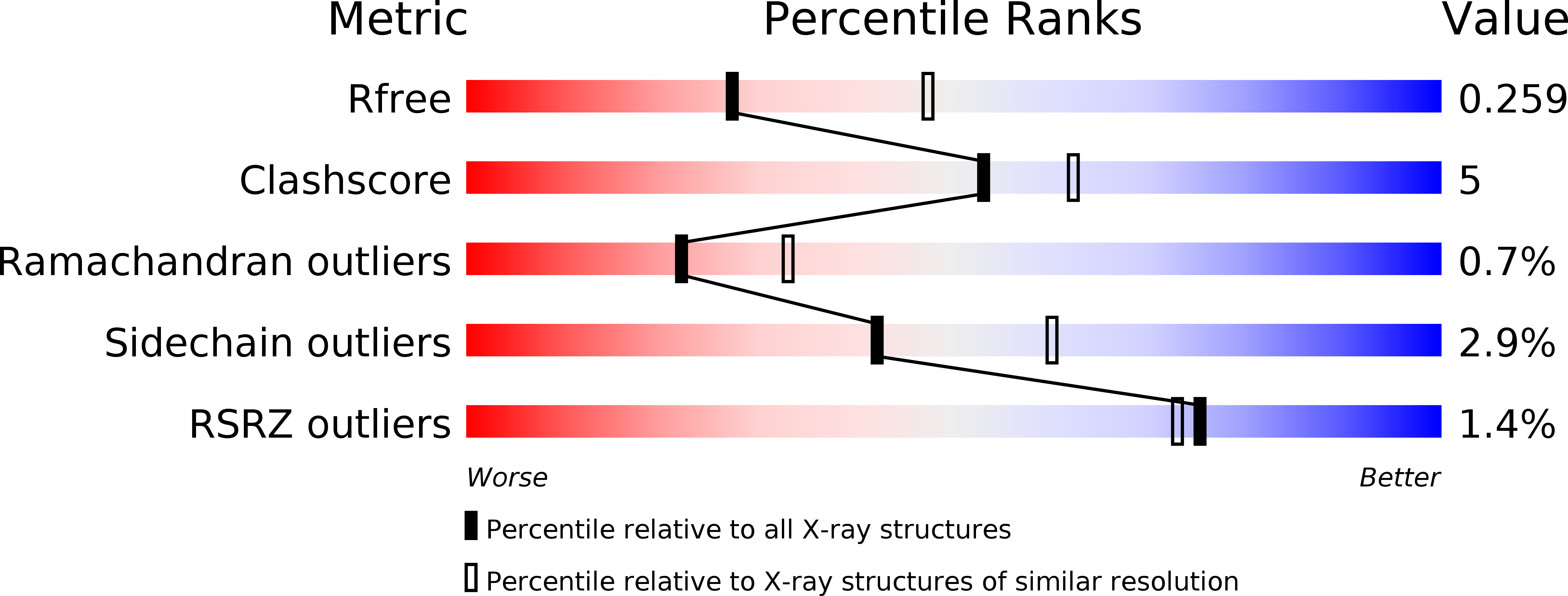
Resolution:
2.65 Å
R-Value Free:
0.26
R-Value Work:
0.18
R-Value Observed:
0.19
Space Group:
P 31