Deposition Date
2015-11-17
Release Date
2015-12-16
Last Version Date
2024-10-30
Entry Detail
PDB ID:
5ESZ
Keywords:
Title:
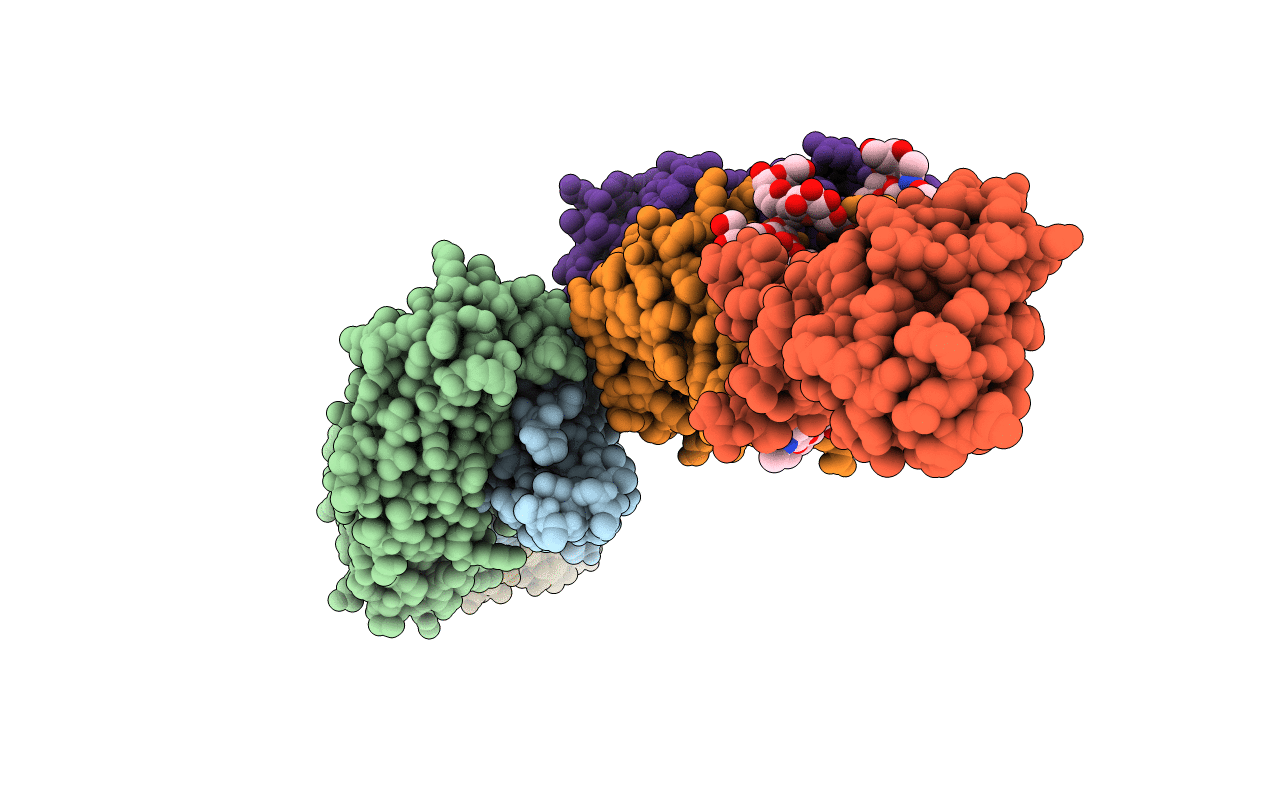
Crystal Structure of Broadly Neutralizing Antibody CH04, Isolated from Donor CH0219, in Complex with Scaffolded Trimeric HIV-1 Env V1V2 Domain from the Clade AE Strain A244
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Haemophilus influenzae (Taxon ID: 71421)
Human immunodeficiency virus 1 (Taxon ID: 11676)
Haemophilus influenzae (Taxon ID: 71421)
Human immunodeficiency virus 1 (Taxon ID: 11676)
Expression System(s):
Method Details:
Experimental Method:
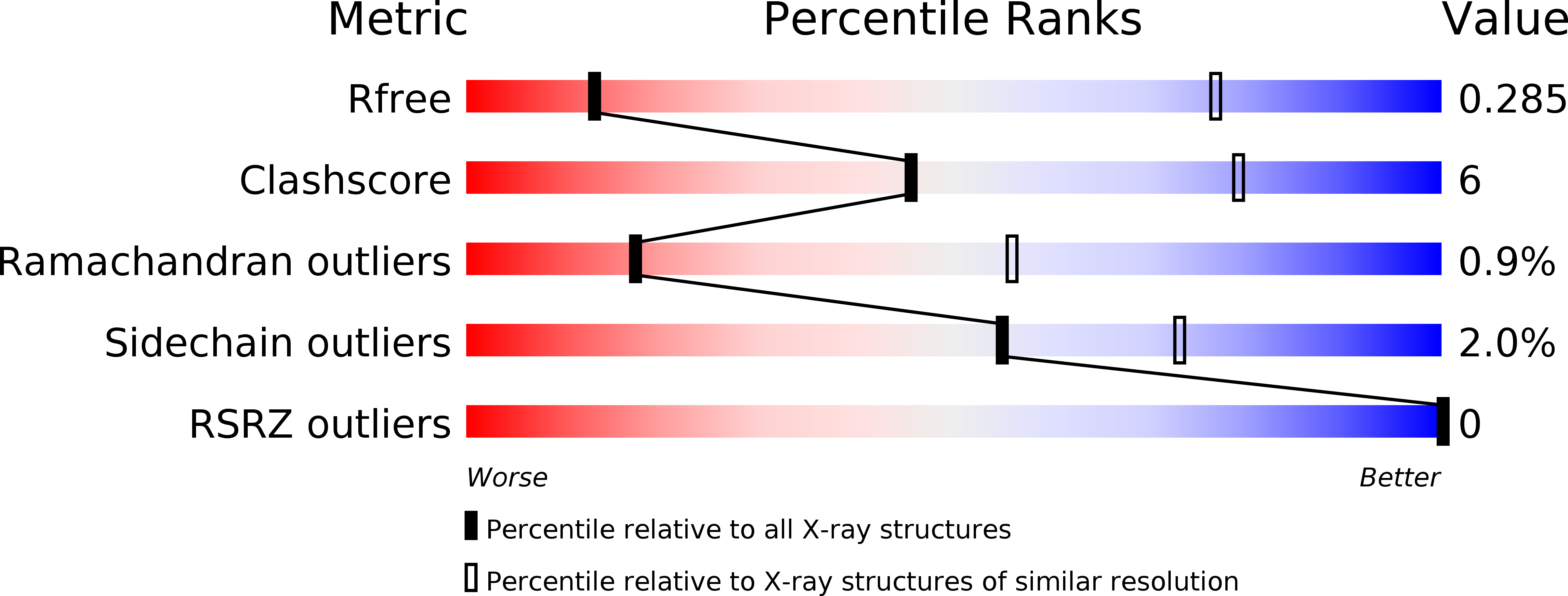
Resolution:
4.19 Å
R-Value Free:
0.28
R-Value Work:
0.25
R-Value Observed:
0.25
Space Group:
P 63