Deposition Date
2015-10-20
Release Date
2016-04-13
Last Version Date
2023-09-27
Entry Detail
PDB ID:
5ED2
Keywords:
Title:
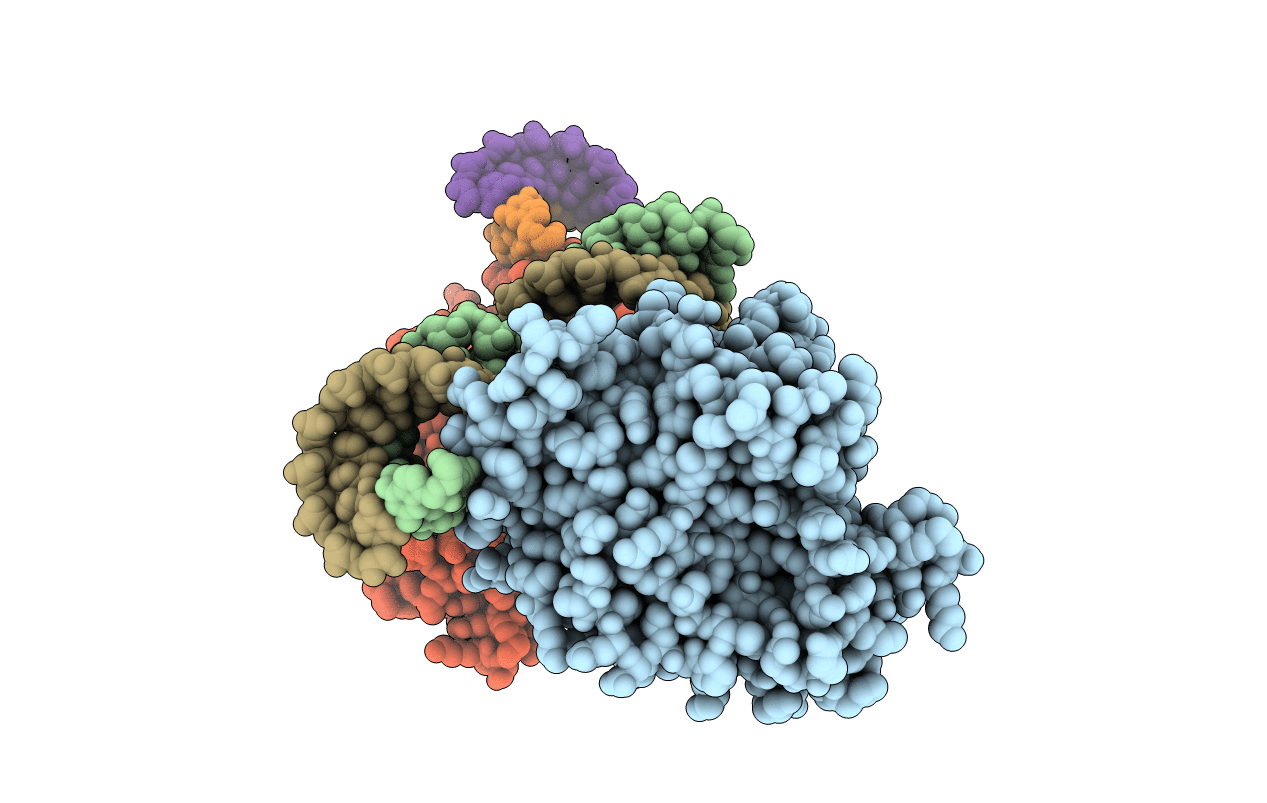
Human Adenosine Deaminase Acting on dsRNA (ADAR2) mutant E488Q bound to dsRNA sequence derived from human GLI1 gene
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
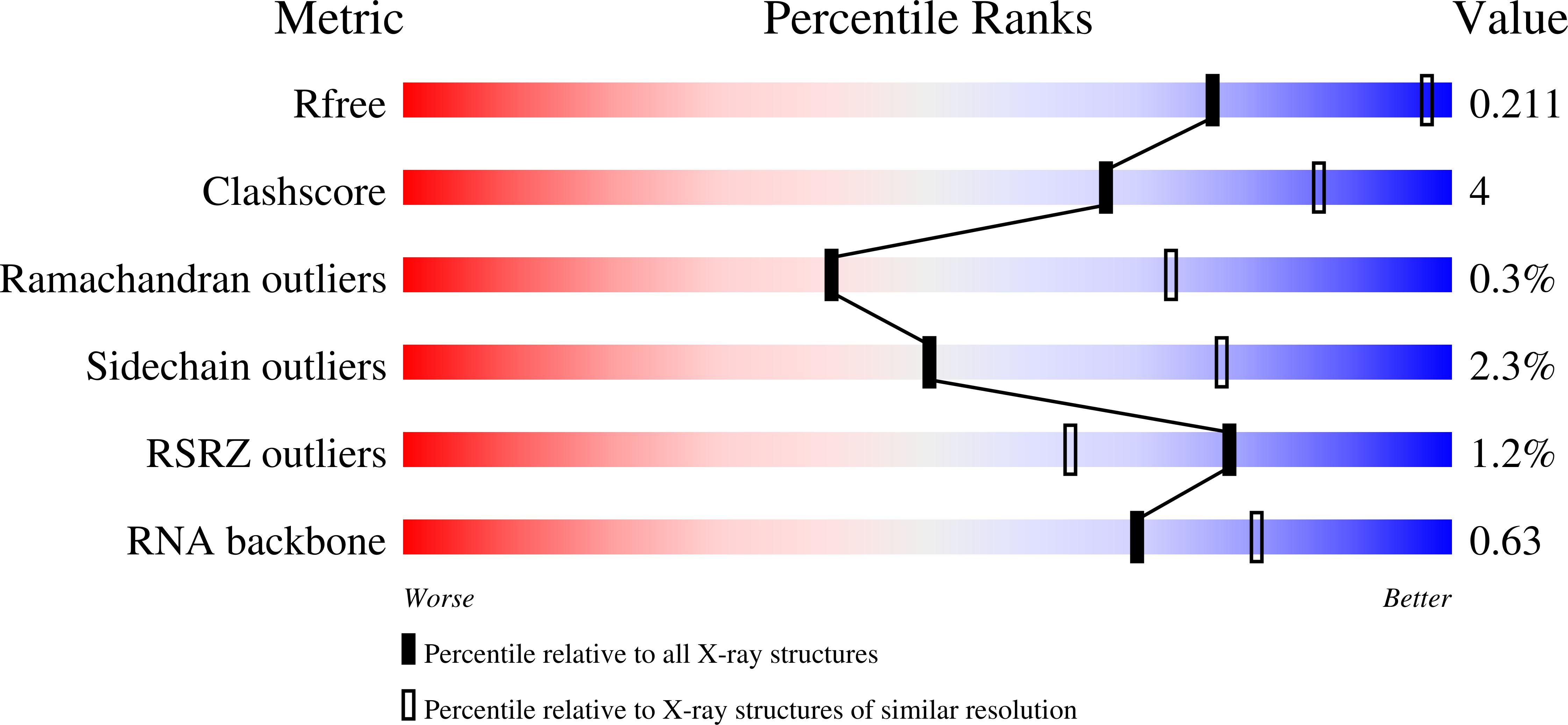
Resolution:
2.95 Å
R-Value Free:
0.20
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 21 21 21