Deposition Date
2015-10-14
Release Date
2016-05-11
Last Version Date
2023-09-27
Entry Detail
PDB ID:
5E91
Keywords:
Title:
TGF-BETA RECEPTOR TYPE 2 KINASE DOMAIN (E431A,R433A,E485A,K488A,R493A,R495A) IN COMPLEX WITH 3-AMINO-6-[4-(2- HYDROXYETHYL)PHENYL]-N-[4-(MORPHOLIN-4-YL)PYRIDIN-3-YL] PYRAZINE-2-CARBOXAMIDE
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
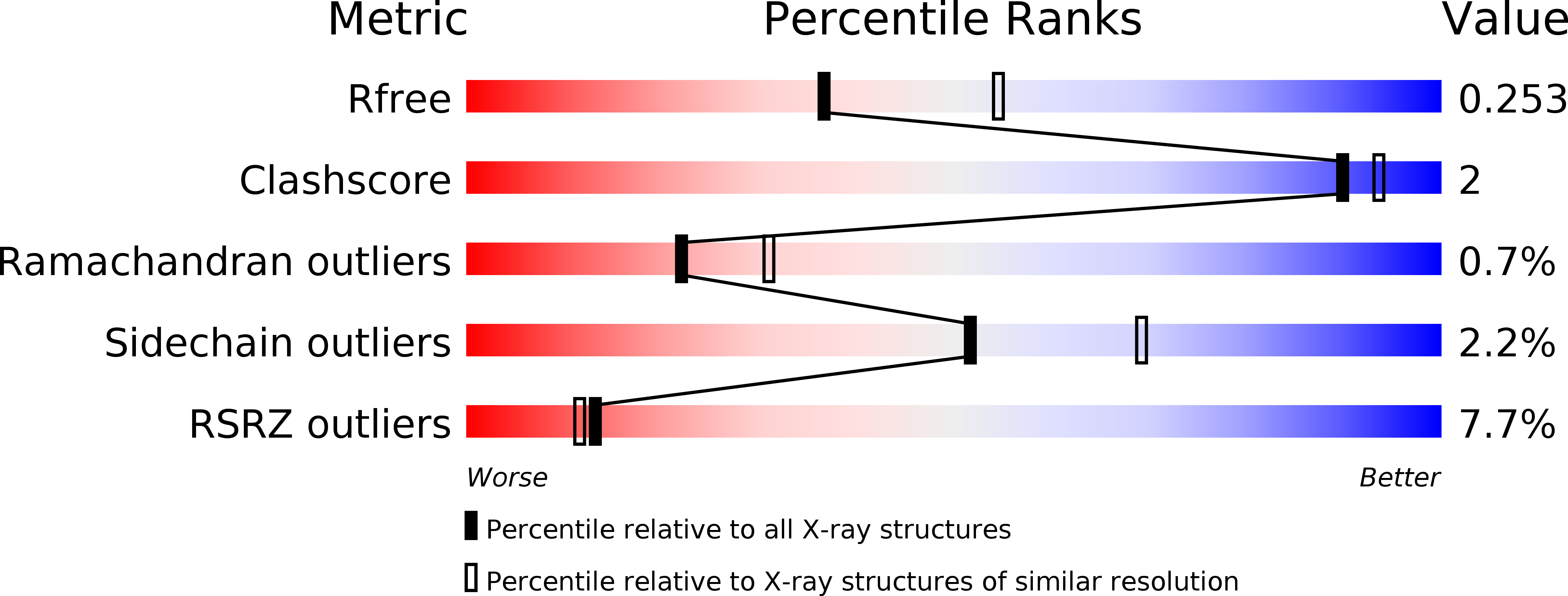
Resolution:
2.42 Å
R-Value Free:
0.24
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 21 21 21