Deposition Date
2015-09-22
Release Date
2015-11-11
Last Version Date
2025-04-02
Entry Detail
PDB ID:
5DW3
Keywords:
Title:
Tryptophan Synthase beta-subunit from Pyrococcus furiosus with product L-tryptophan non-covalently bound in the active site
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
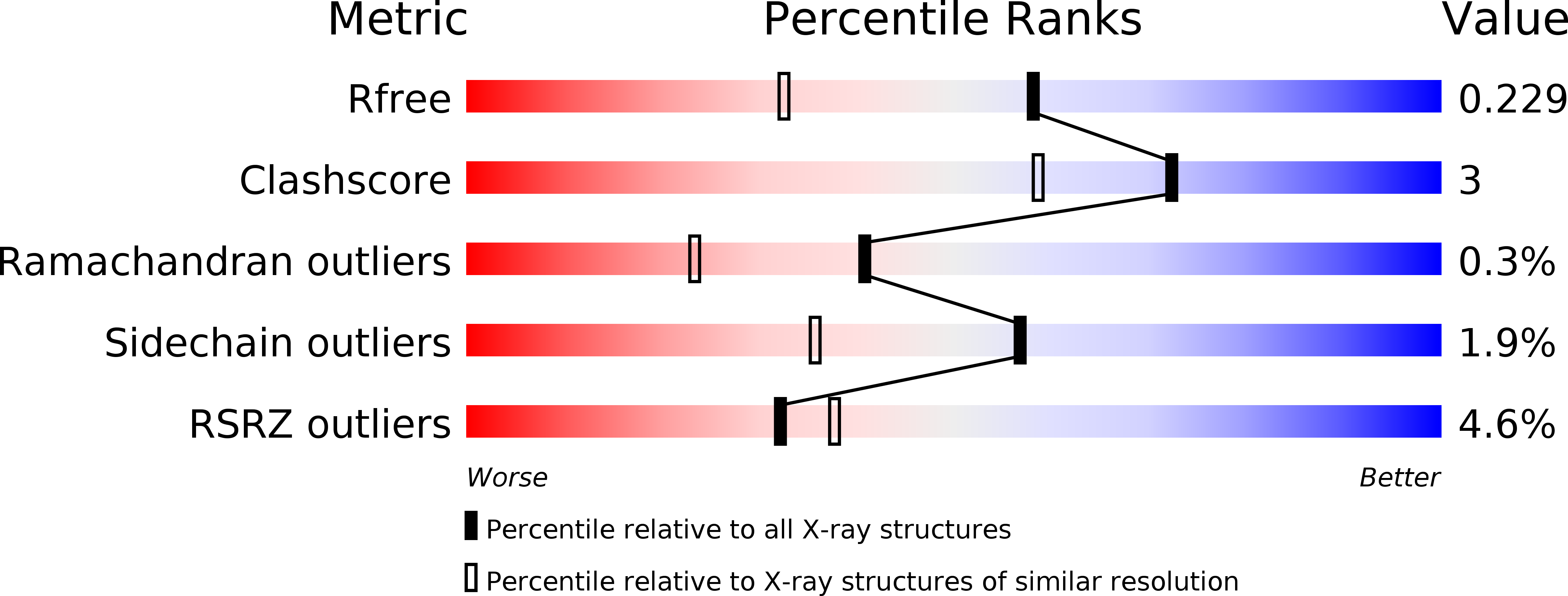
Resolution:
1.74 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 21 21 21