Deposition Date
2015-08-23
Release Date
2016-10-05
Last Version Date
2024-12-25
Entry Detail
PDB ID:
5DC2
Keywords:
Title:
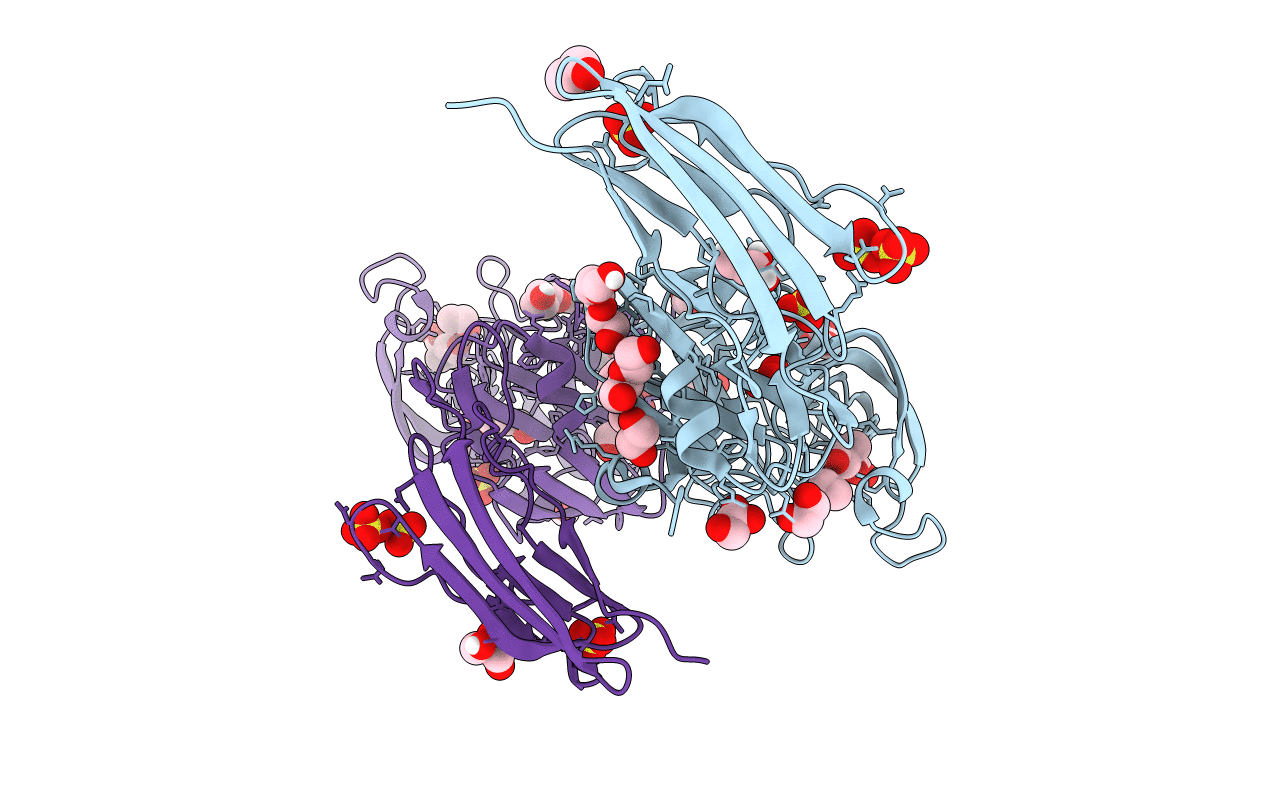
X-RAY CRYSTAL STRUCTURE OF A ENZYMATICALLY DEGRADED BIAPENEM-ADDUCT OF L,D-TRANSPEPTIDASE 2 FROM MYCOBACTERIUM TUBERCULOSIS
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
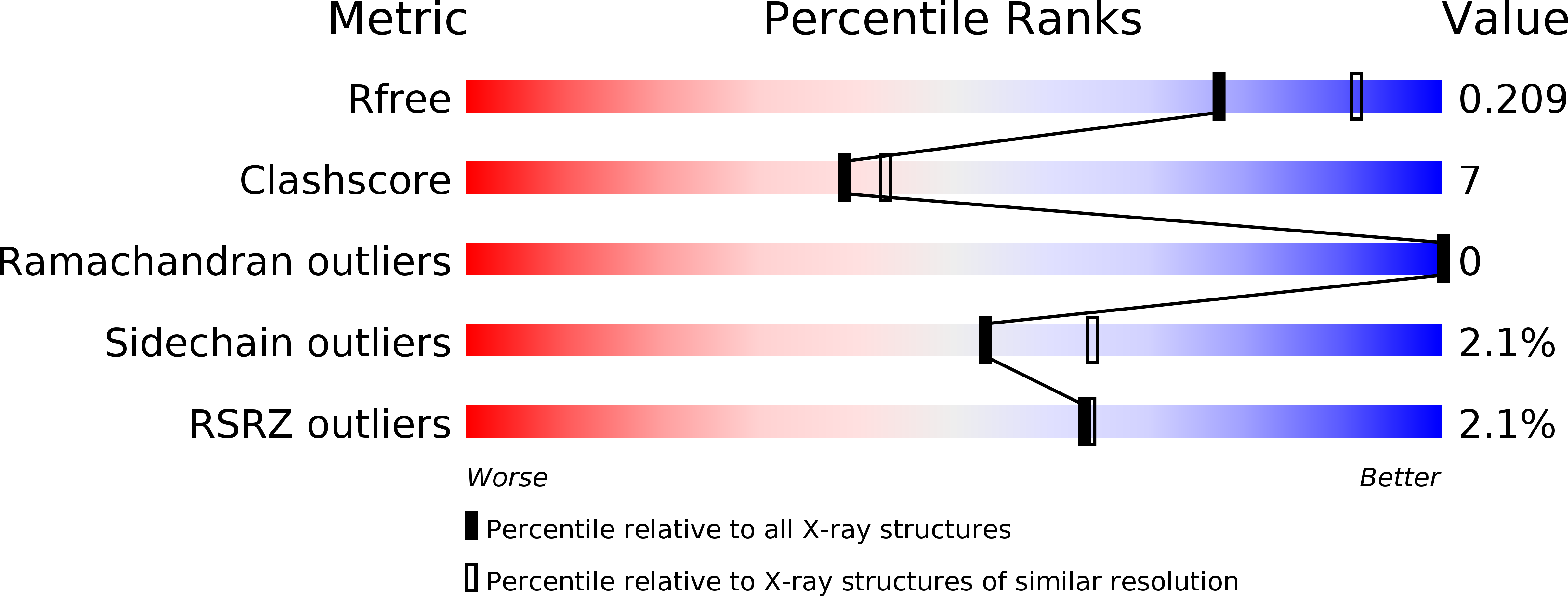
Resolution:
2.18 Å
R-Value Free:
0.20
R-Value Work:
0.15
R-Value Observed:
0.16
Space Group:
P 1 21 1