Deposition Date
2015-07-22
Release Date
2015-08-12
Last Version Date
2025-04-09
Entry Detail
PDB ID:
5CR5
Keywords:
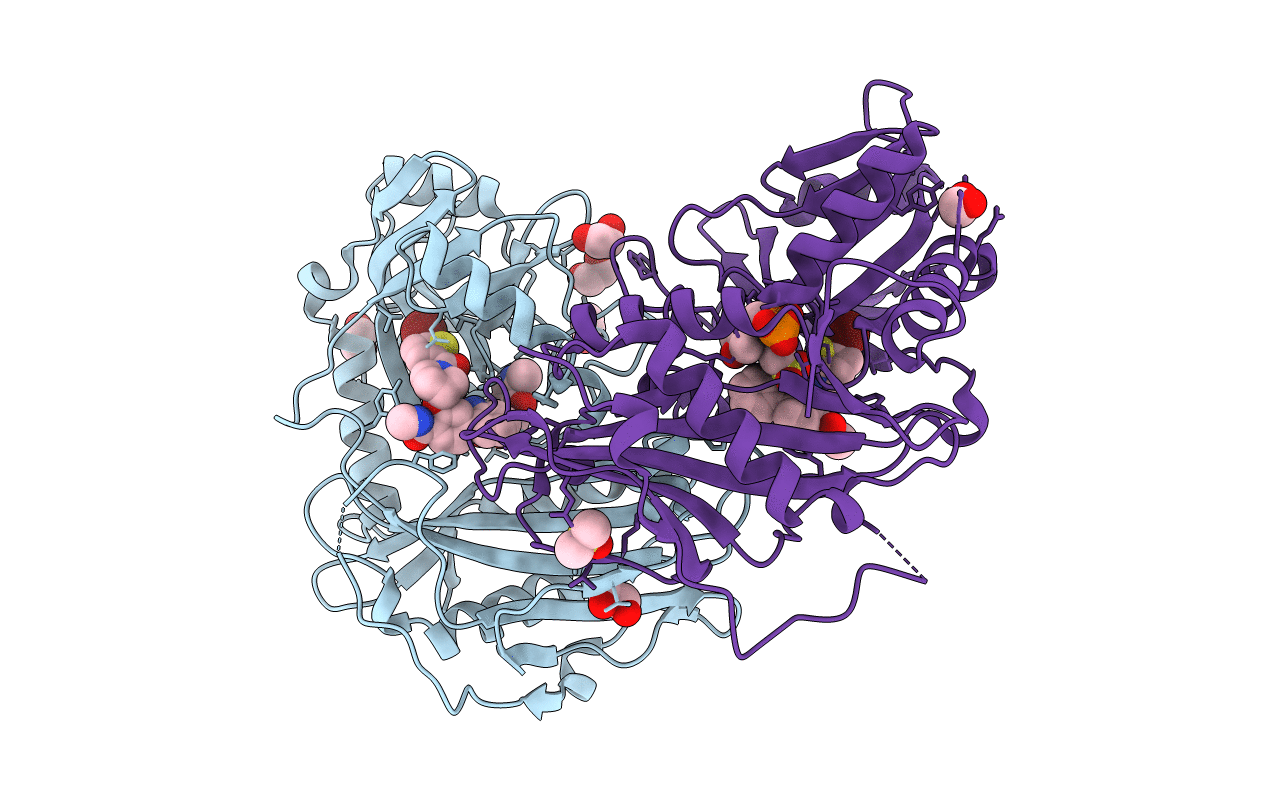
Title:
X-RAY CRYSTAL STRUCTURE AT 1.61A RESOLUTION OF HUMAN MITOCHONDRIAL BRANCHED CHAIN AMINOTRANSFERASE (BCATM) COMPLEXED WITH A BIPHENYL PYRROLIDINE ETHER COMPOUND AND AN INTERNAL ALDIMINE LINKED PLP COFACTOR.
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.61 Å
R-Value Free:
0.19
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 21 21 21


