Deposition Date
2015-07-09
Release Date
2015-12-02
Last Version Date
2024-06-26
Entry Detail
PDB ID:
5CGH
Keywords:
Title:
Yeast 20S proteasome beta5-G48C mutant in complex with alpha-chloroacetamide 5
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Saccharomyces cerevisiae S288C (Taxon ID: 559292)
Saccharomyces cerevisiae S288C (Taxon ID: 559292)
Method Details:
Experimental Method:
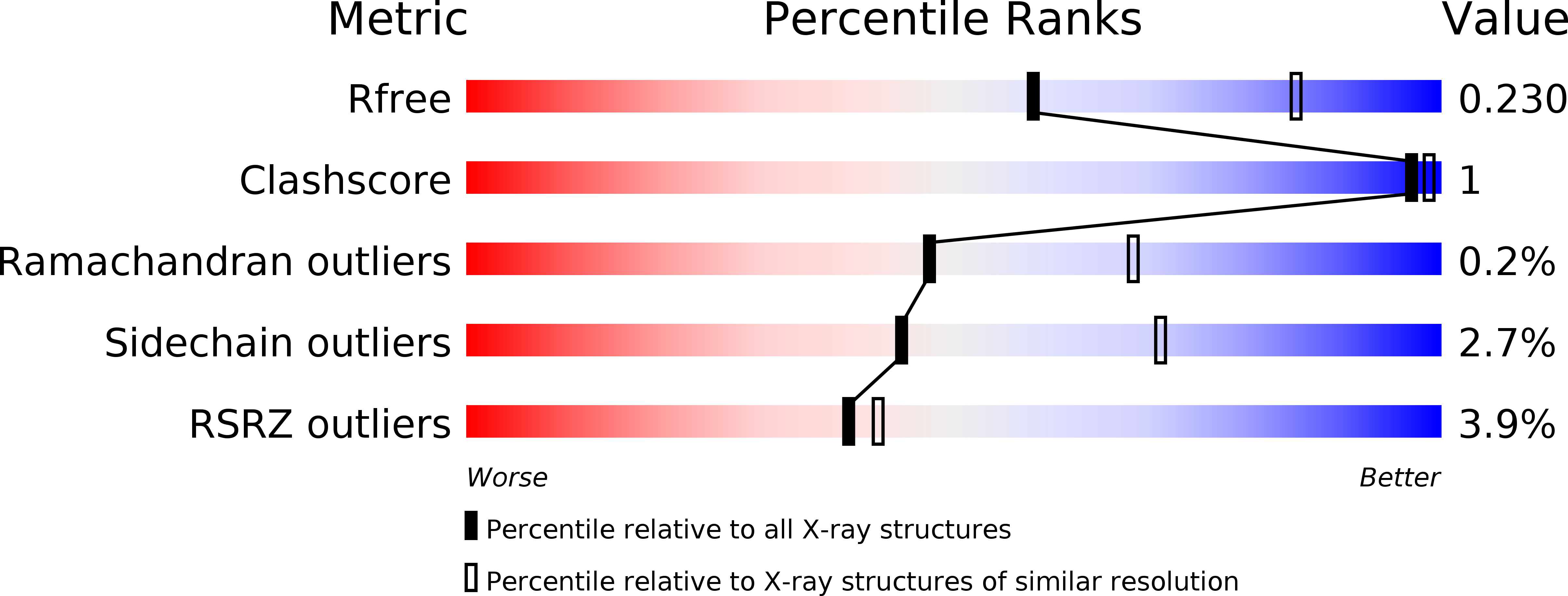
Resolution:
2.50 Å
R-Value Free:
0.22
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 1 21 1