Deposition Date
2015-06-24
Release Date
2015-09-30
Last Version Date
2023-09-27
Entry Detail
PDB ID:
5C7R
Keywords:
Title:
Revealing surface waters on an antifreeze protein by fusion protein crystallography
Biological Source:
Source Organism(s):
Escherichia coli O157:H7 (Taxon ID: 83334)
Zoarces americanus (Taxon ID: 8199)
Zoarces americanus (Taxon ID: 8199)
Expression System(s):
Method Details:
Experimental Method:
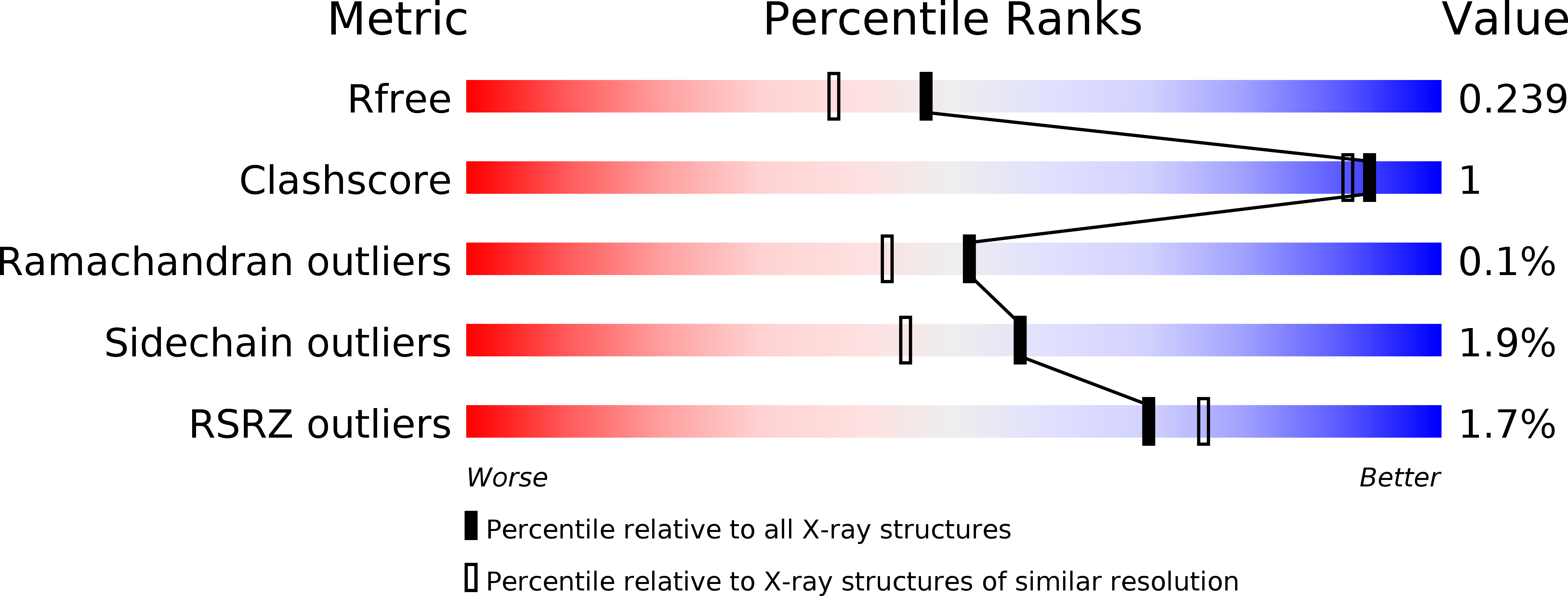
Resolution:
1.94 Å
R-Value Free:
0.23
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 1 21 1