Deposition Date
2015-06-03
Release Date
2016-07-13
Last Version Date
2025-04-09
Entry Detail
PDB ID:
5BTT
Keywords:
Title:
Switching GFP fluorescence using genetically encoded phenyl azide chemistry through two different non-native post-translational modifications routes at the same position.
Biological Source:
Source Organism(s):
Aequorea victoria (Taxon ID: 6100)
Expression System(s):
Method Details:
Experimental Method:
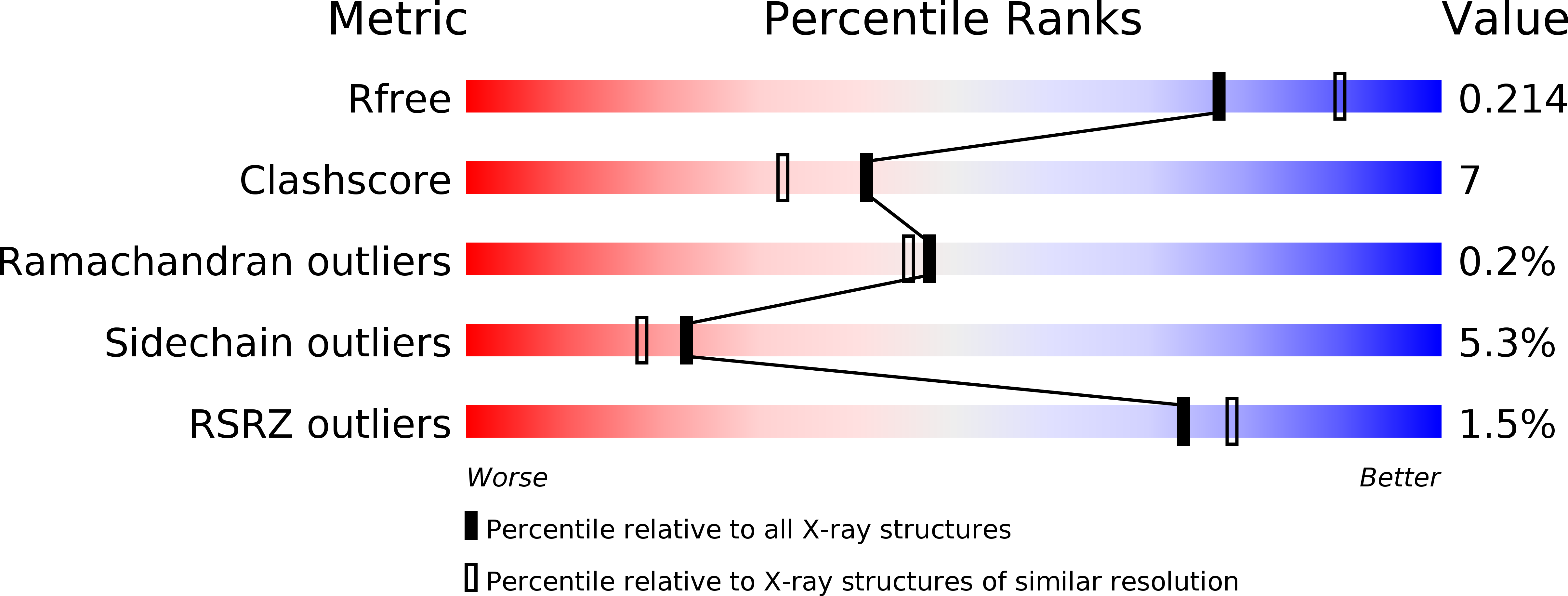
Resolution:
2.14 Å
R-Value Free:
0.20
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
P 43 21 2