Deposition Date
2015-05-28
Release Date
2015-12-30
Last Version Date
2024-01-10
Entry Detail
PDB ID:
5BPJ
Keywords:
Title:
All Three Ca(2+)-binding Loops of Light-sensitive Ctenophore Photoprotein Berovin Bind Magnesium Ions: The Spatial Structure of Mg(2+)-loaded Apo-berovin
Biological Source:
Source Organism(s):
Beroe abyssicola (Taxon ID: 320166)
Expression System(s):
Method Details:
Experimental Method:
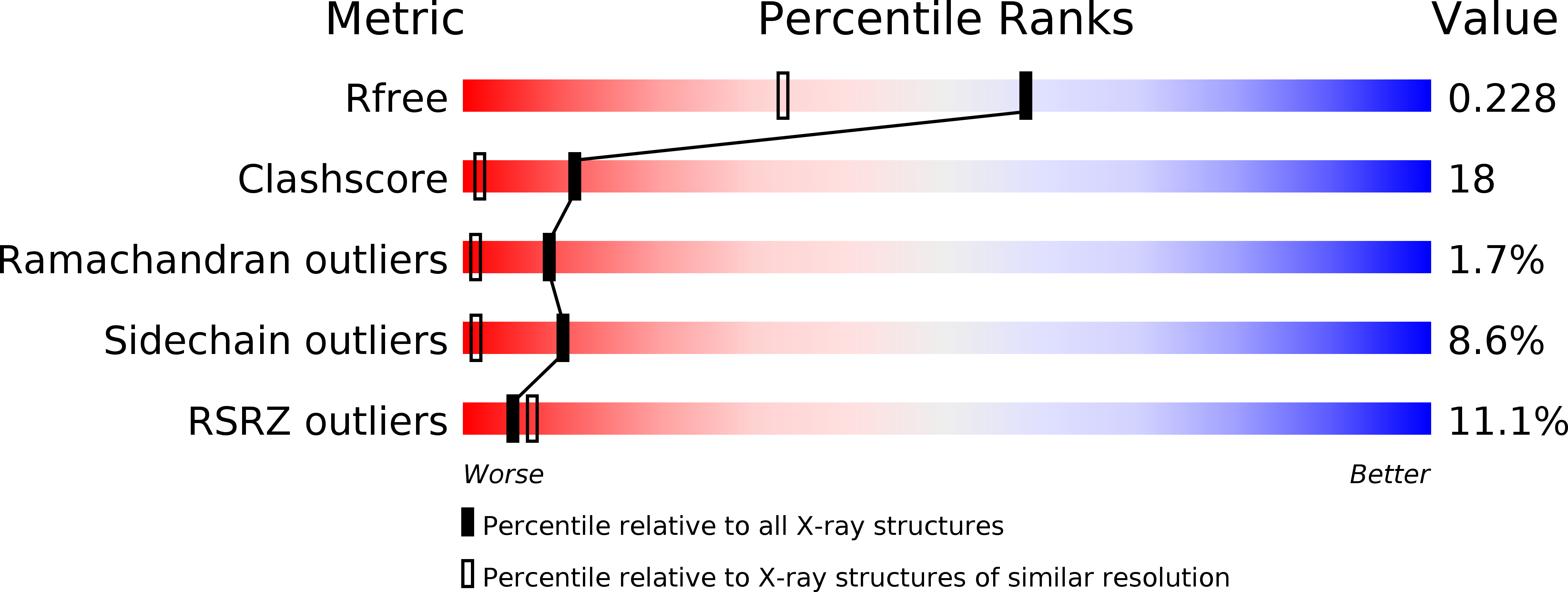
Resolution:
1.76 Å
R-Value Free:
0.24
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
C 1 2 1