Deposition Date
2015-11-11
Release Date
2016-07-27
Last Version Date
2024-11-20
Entry Detail
PDB ID:
5B0W
Keywords:
Title:
Crystal structure of the 11-cis isomer of pharaonis halorhodopsin in the absence of halide ions
Biological Source:
Source Organism(s):
Natronomonas pharaonis DSM 2160 (Taxon ID: 348780)
Method Details:
Experimental Method:
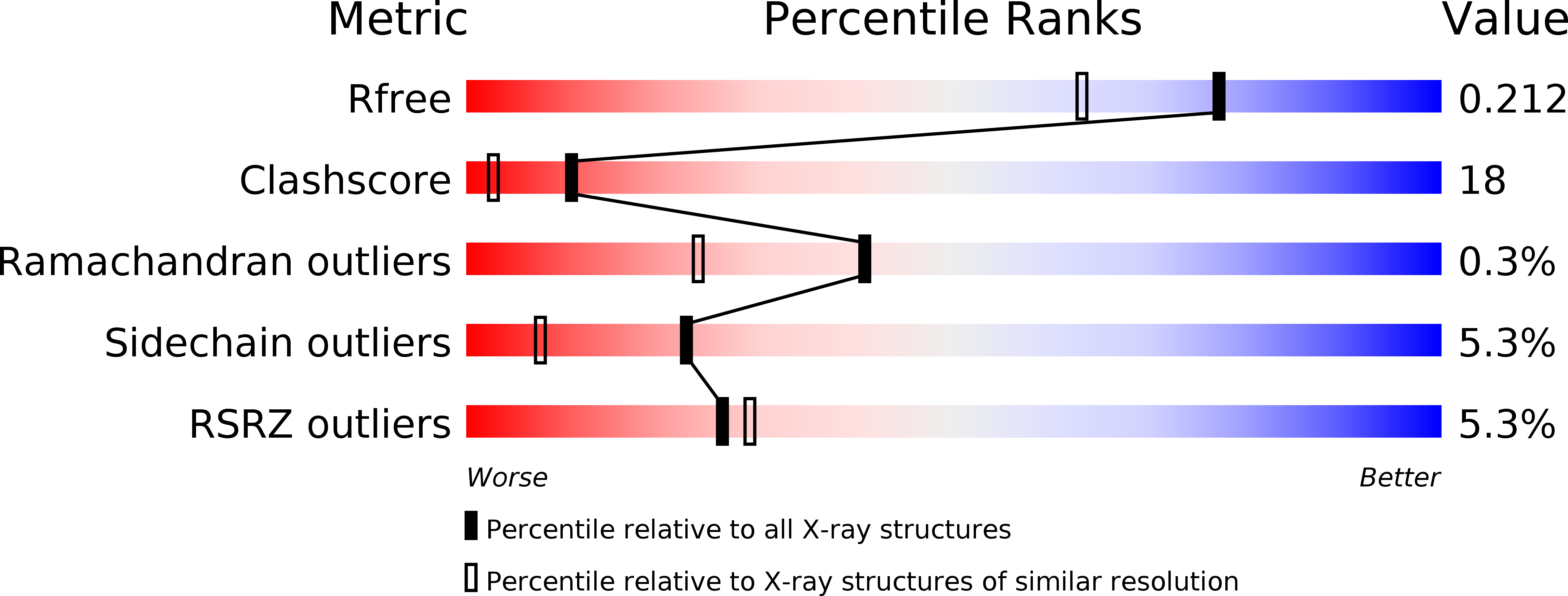
Resolution:
1.70 Å
R-Value Free:
0.21
R-Value Work:
0.20
Space Group:
C 1 2 1