Deposition Date
2015-09-10
Release Date
2015-12-09
Last Version Date
2024-01-10
Entry Detail
PDB ID:
5AO9
Keywords:
Title:
The structure of a novel thermophilic esterase from the Planctomycetes species, Thermogutta terrifontis, Est2-native
Biological Source:
Source Organism:
THERMOGUTTA TERRIFONTIS (Taxon ID: 1331910)
Host Organism:
Method Details:
Experimental Method:
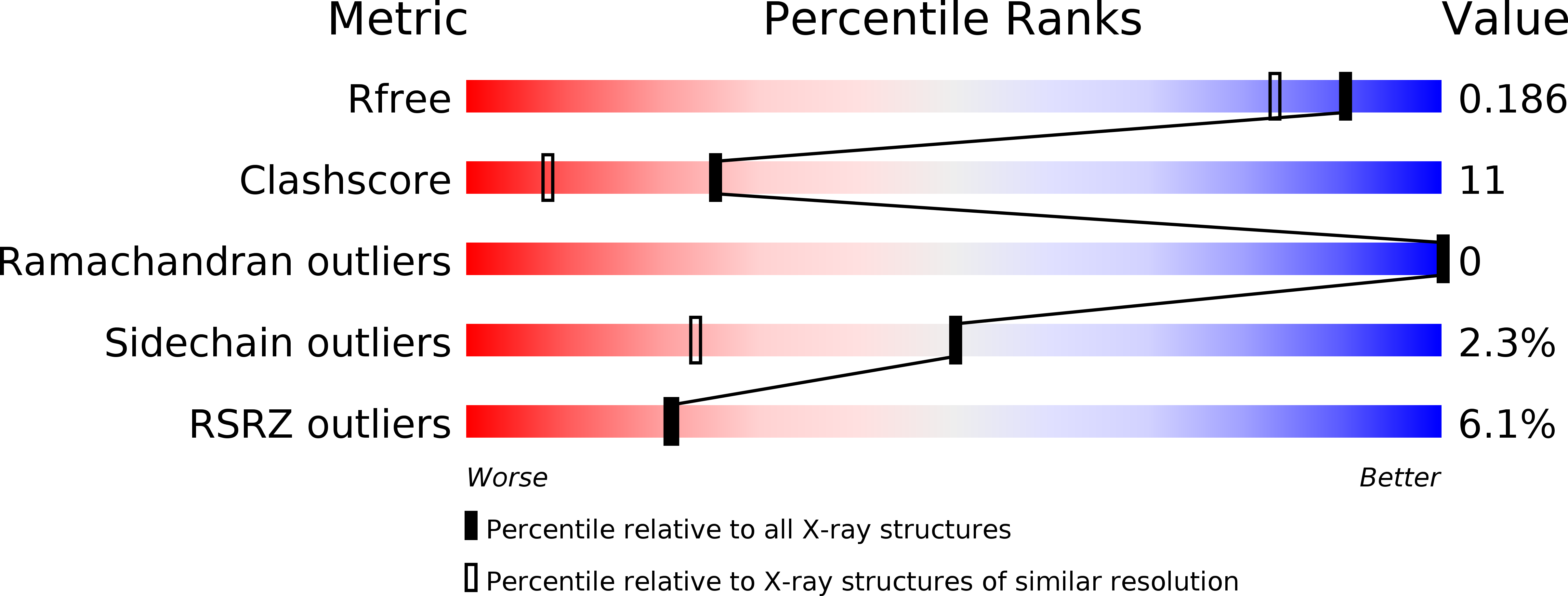
Resolution:
1.58 Å
R-Value Free:
0.17
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 21 21 21