Deposition Date
2015-02-06
Release Date
2015-06-03
Last Version Date
2024-01-10
Entry Detail
PDB ID:
5AHS
Keywords:
Title:
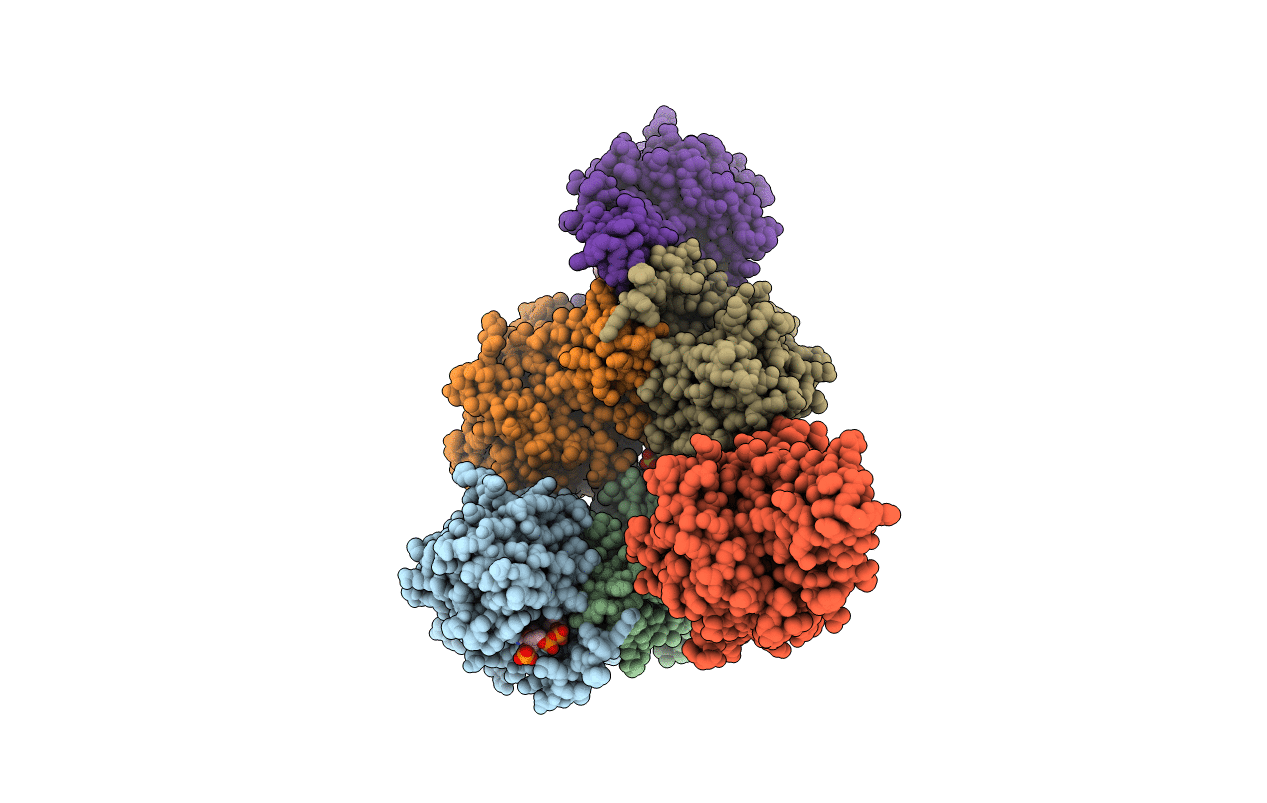
3-Sulfinopropionyl-Coenzyme A (3SP-CoA) desulfinase from Advenella mimgardefordensis DPN7T: holo crystal structure with the substrate analog succinyl-CoA
Biological Source:
Source Organism(s):
ADVENELLA MIMIGARDEFORDENSIS DPN7 (Taxon ID: 1247726)
Expression System(s):
Method Details:
Experimental Method:
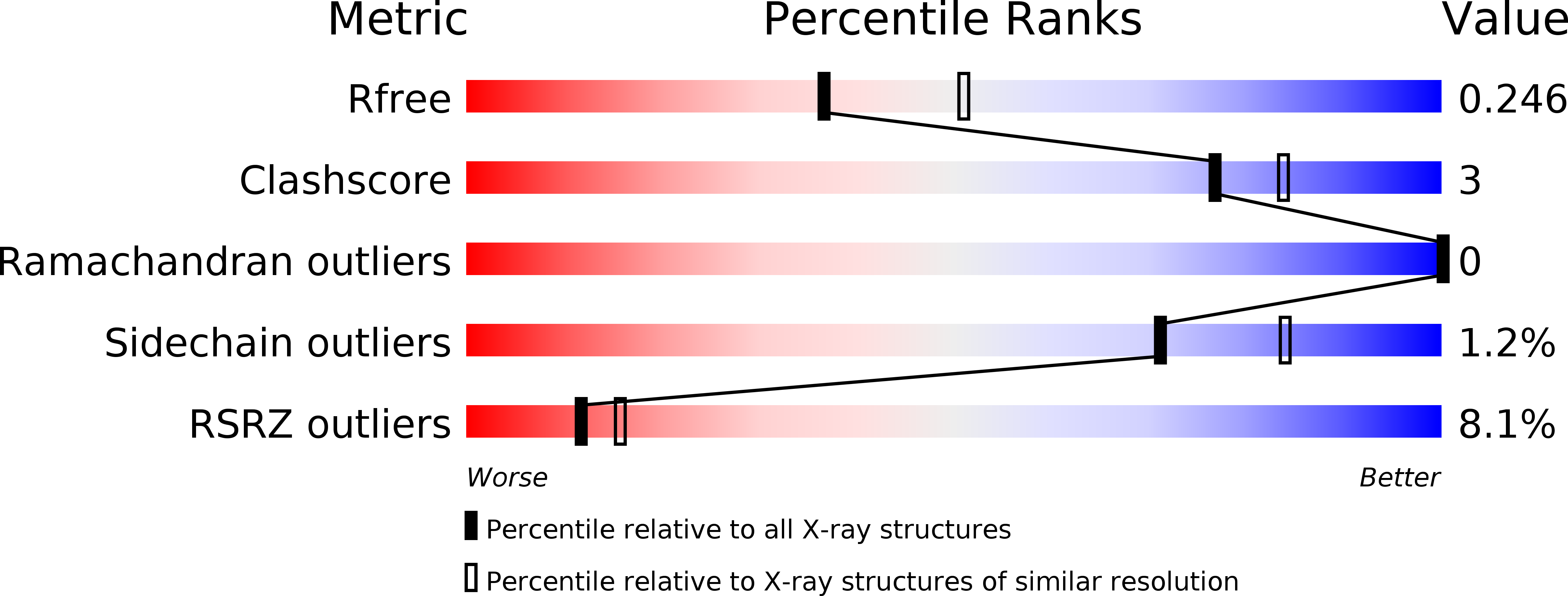
Resolution:
2.30 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
P 21 21 2