Deposition Date
2015-08-15
Release Date
2015-12-16
Last Version Date
2024-05-01
Entry Detail
PDB ID:
5ACC
Keywords:
Title:
A Novel Oral Selective Estrogen Receptor Down-regulator, AZD9496, drives Tumour Growth Inhibition in Estrogen Receptor positive and ESR1 Mutant Models
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
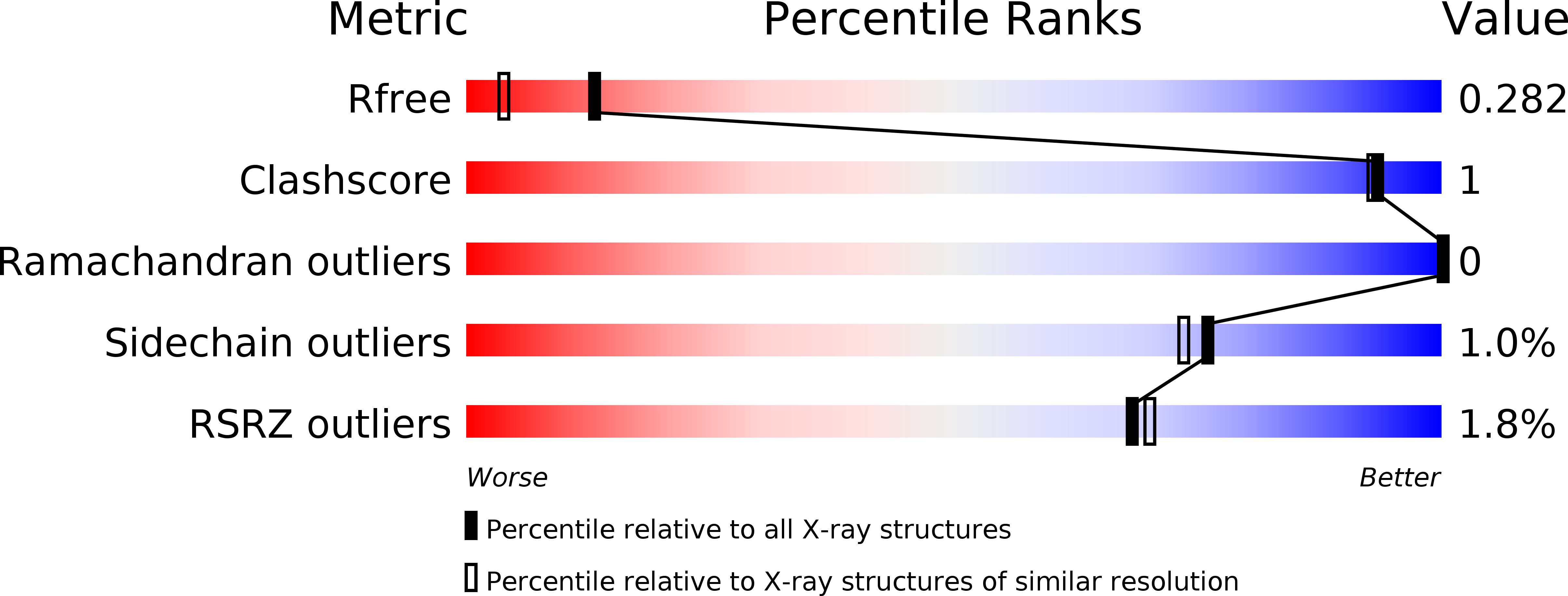
Resolution:
1.88 Å
R-Value Free:
0.26
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 65 2 2