Deposition Date
2015-07-15
Release Date
2015-11-18
Last Version Date
2024-11-06
Entry Detail
PDB ID:
5A8G
Keywords:
Title:
Crystal structure of the wild-type Staphylococcus aureus N- acetylneurminic acid lyase in complex with fluoropyruvate
Biological Source:
Source Organism(s):
STAPHYLOCOCCUS AUREUS SUBSP. AUREUS NCTC 8325 (Taxon ID: 93061)
Expression System(s):
Method Details:
Experimental Method:
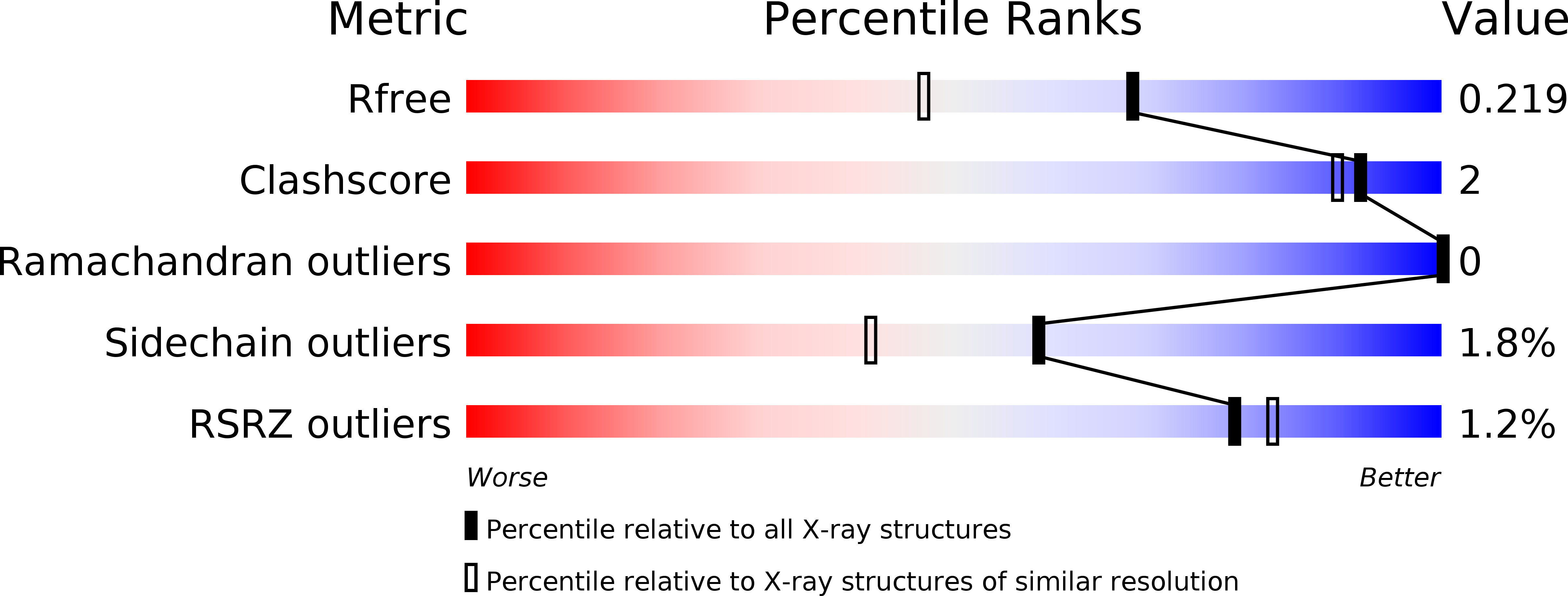
Resolution:
1.72 Å
R-Value Free:
0.21
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
C 2 2 21