Deposition Date
2015-07-03
Release Date
2015-09-23
Last Version Date
2024-01-10
Entry Detail
PDB ID:
5A78
Keywords:
Title:
Crystal structure of the homing endonuclease I-CvuI in complex with I- CreI target (C1221) in the presence of 2 mM Mg revealing DNA not cleaved
Biological Source:
Source Organism(s):
CHLORELLA VULGARIS (Taxon ID: 3077)
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
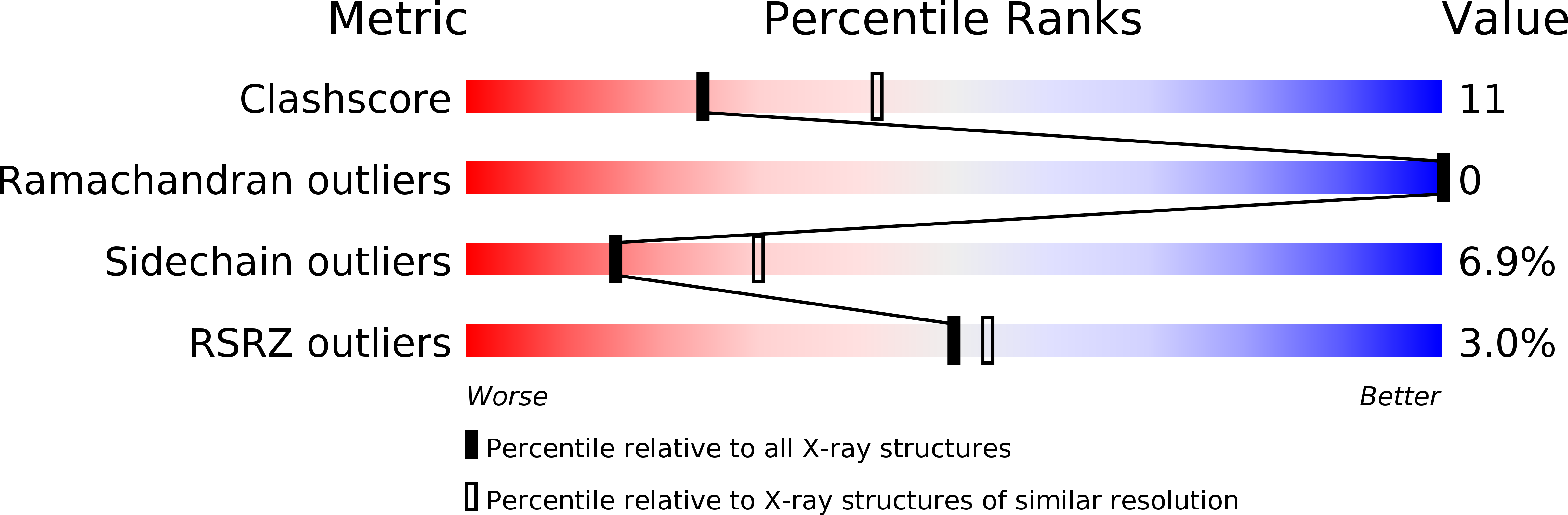
Resolution:
2.50 Å
R-Value Free:
0.28
R-Value Work:
0.21
R-Value Observed:
0.22
Space Group:
P 1 21 1