Deposition Date
2015-04-23
Release Date
2015-08-19
Last Version Date
2024-01-10
Entry Detail
PDB ID:
5A0V
Keywords:
Title:
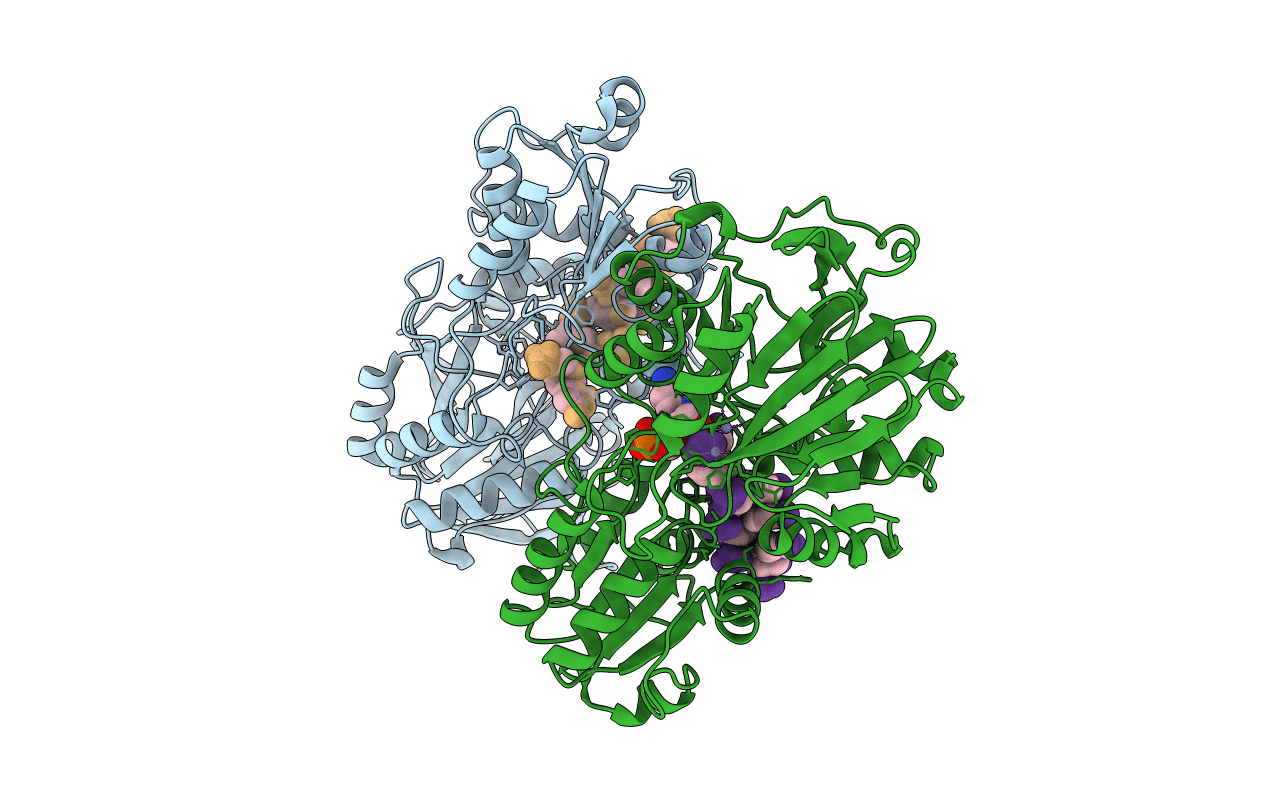
Catalysis and 5' end sensing by ribonuclease RNase J of the metallo- beta-lactamase family
Biological Source:
Source Organism(s):
STREPTOMYCES COELICOLOR A3(2) (Taxon ID: 100226)
Expression System(s):
Method Details:
Experimental Method:
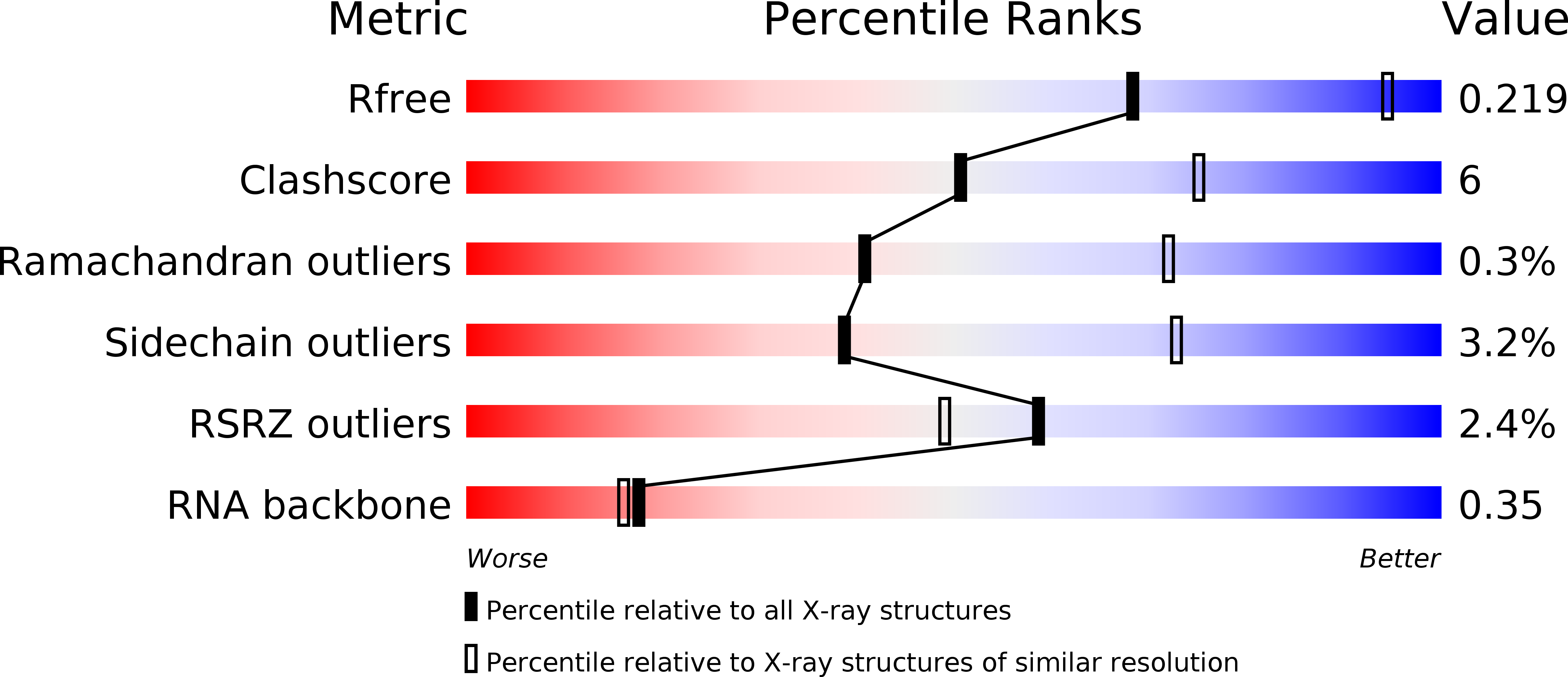
Resolution:
2.80 Å
R-Value Free:
0.20
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 43 2 2