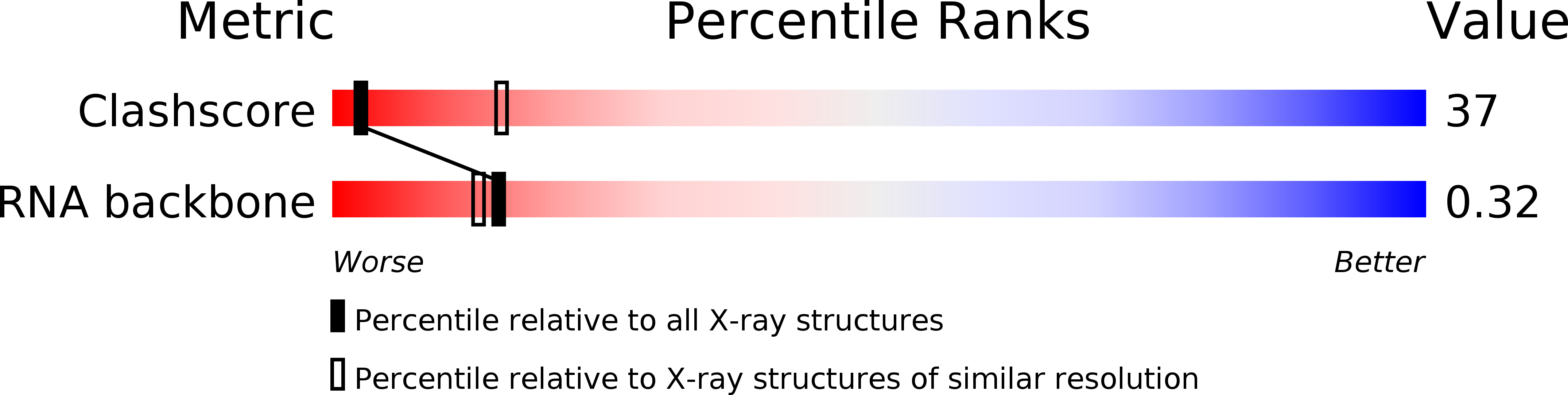
Deposition Date
1987-11-06
Release Date
1987-11-06
Last Version Date
2023-09-27
Entry Detail
PDB ID:
4TRA
Keywords:
Title:
RESTRAINED REFINEMENT OF TWO CRYSTALLINE FORMS OF YEAST ASPARTIC ACID AND PHENYLALANINE TRANSFER RNA CRYSTALS
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 4932)
Method Details:
Experimental Method:
Resolution:
3.00 Å
R-Value Observed:
0.17
Space Group:
P 21 2 21