Deposition Date
2015-05-22
Release Date
2016-06-29
Last Version Date
2024-11-20
Entry Detail
PDB ID:
4ZZA
Keywords:
Title:
Raffinose and panose binding protein from Bifidobacterium animalis subsp. lactis Bl-04, bound with raffinose, selenomethionine derivative
Biological Source:
Source Organism:
Bifidobacterium animalis subsp. lactis Bl-04 (Taxon ID: 580050)
Host Organism:
Method Details:
Experimental Method:
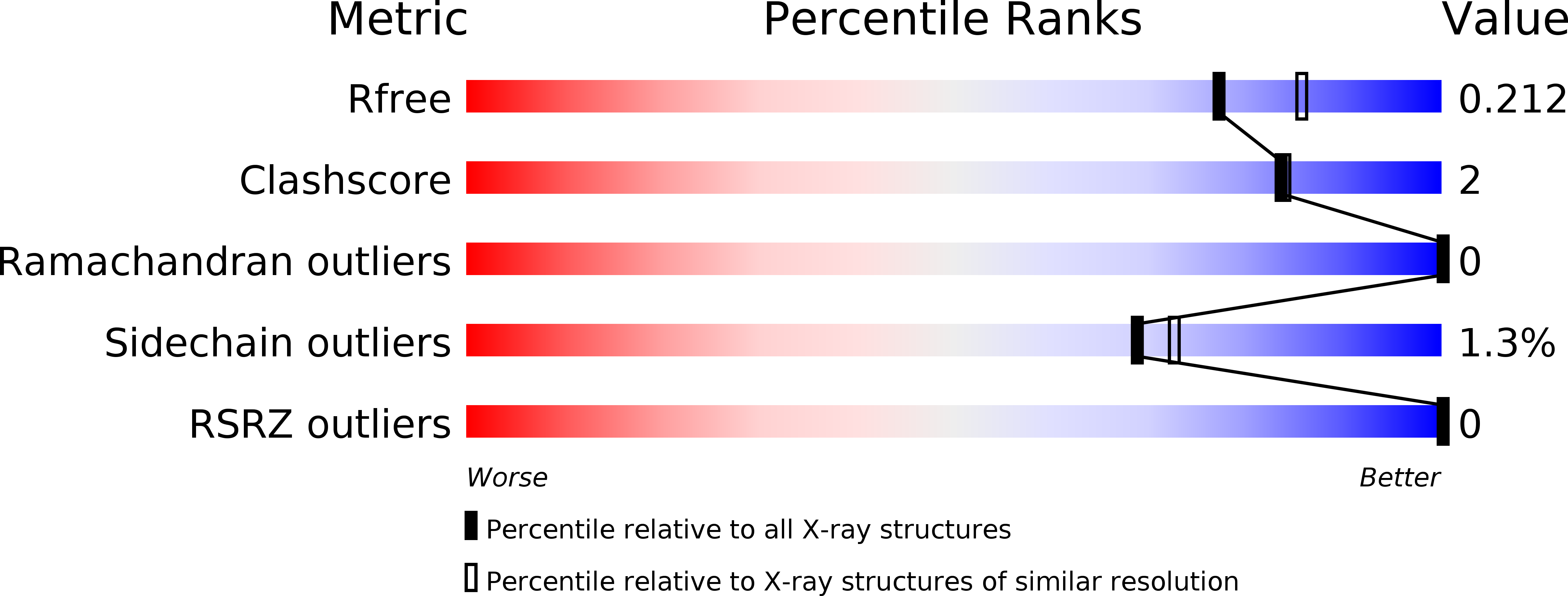
Resolution:
2.02 Å
R-Value Free:
0.21
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
P 21 21 21