Deposition Date
2015-05-11
Release Date
2015-10-28
Last Version Date
2023-09-27
Entry Detail
PDB ID:
4ZQU
Keywords:
Title:
CdiA-CT/CdiI toxin and immunity complex from Yersinia pseudotuberculosis
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
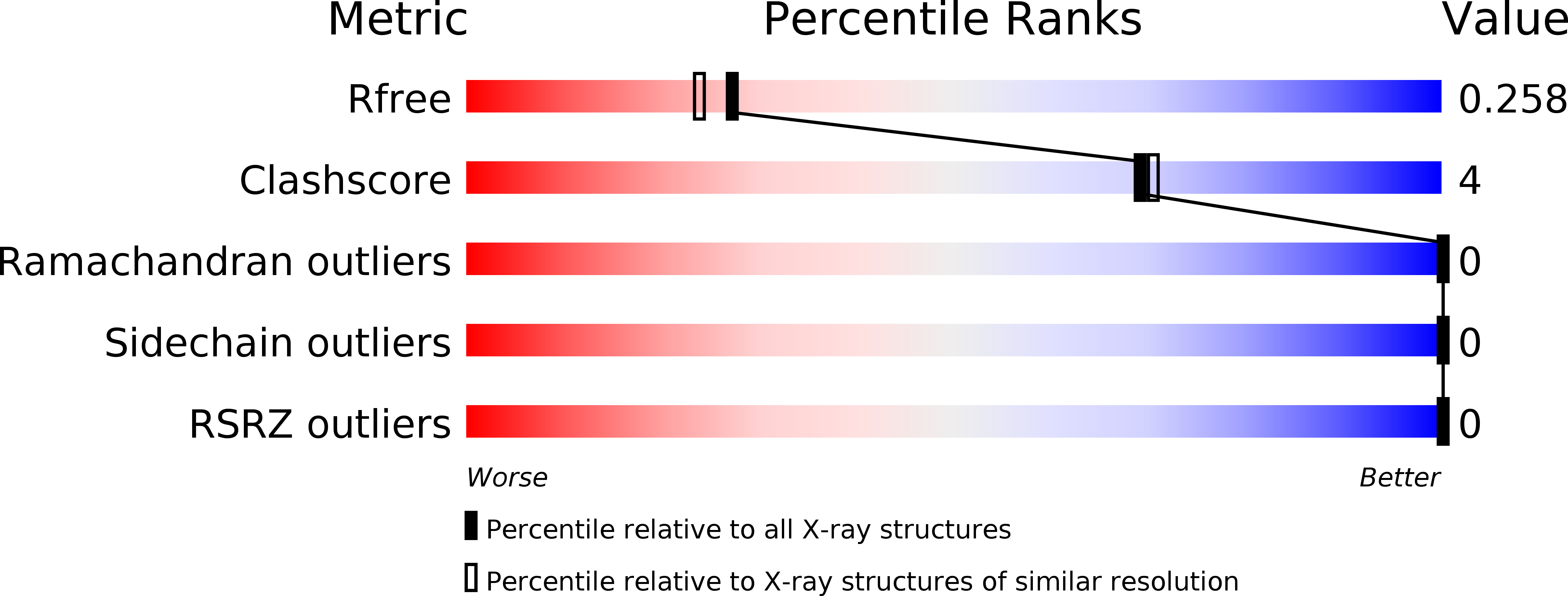
Resolution:
2.09 Å
R-Value Free:
0.25
R-Value Work:
0.20
R-Value Observed:
0.21
Space Group:
C 1 2 1