Deposition Date
2015-05-04
Release Date
2016-04-27
Last Version Date
2024-03-20
Entry Detail
PDB ID:
4ZMR
Keywords:
Title:
Structural characterization of the full-length response regulator spr1814 in complex with a phosphate analogue reveals a novel conformational plasticity of the linker region
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
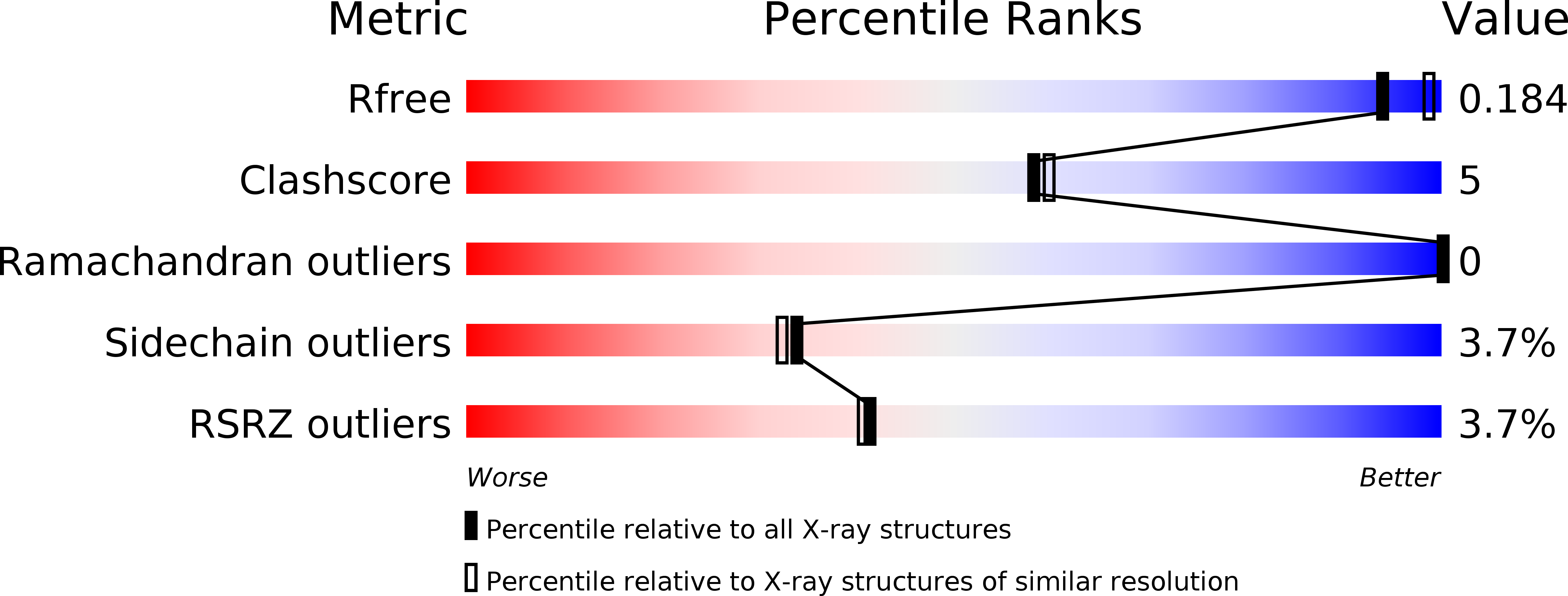
Resolution:
2.00 Å
R-Value Free:
0.22
R-Value Work:
0.17
R-Value Observed:
0.18
Space Group:
P 1 21 1