Deposition Date
2015-05-02
Release Date
2015-12-09
Last Version Date
2024-03-20
Entry Detail
PDB ID:
4ZM6
Keywords:
Title:
A unique GCN5-related glucosamine N-acetyltransferase region exist in the fungal multi-domain GH3 beta-N-acetylglucosaminidase
Biological Source:
Source Organism(s):
Rhizomucor miehei CAU432 (Taxon ID: 1031333)
Expression System(s):
Method Details:
Experimental Method:
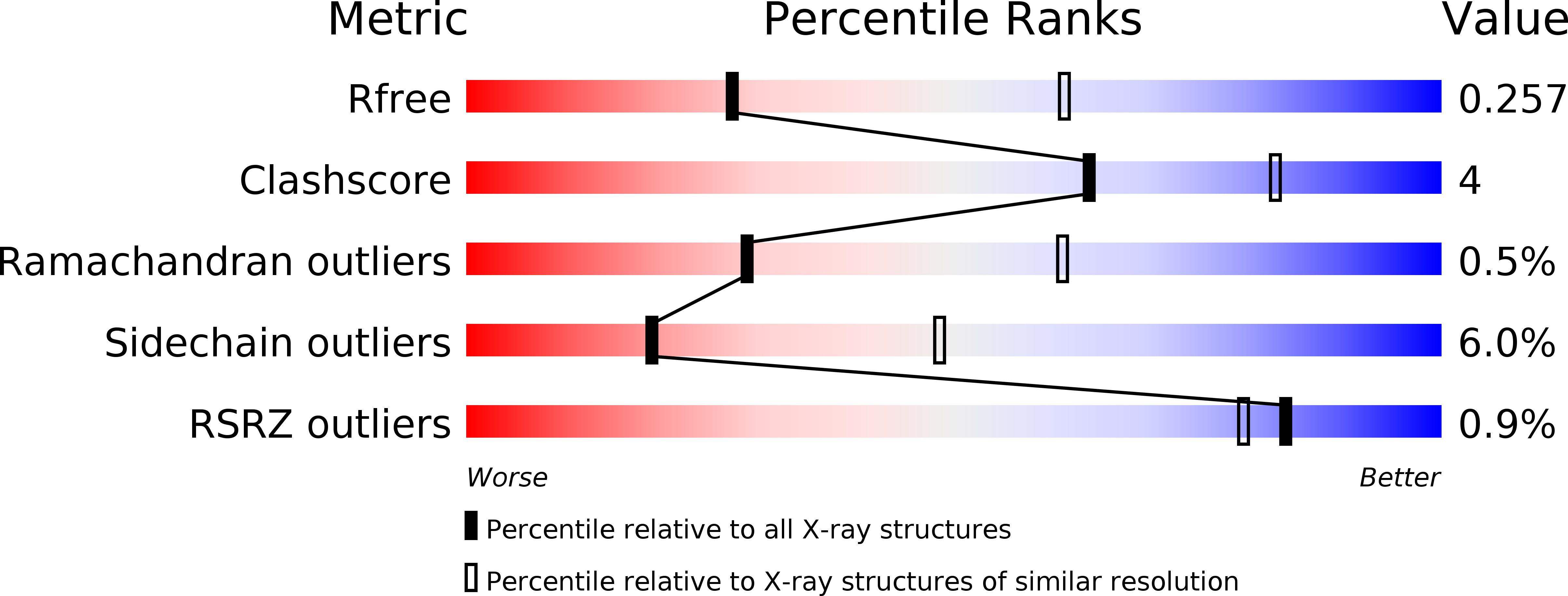
Resolution:
2.80 Å
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.23
Space Group:
P 41 21 2