Deposition Date
2015-04-06
Release Date
2015-09-02
Last Version Date
2023-09-27
Entry Detail
PDB ID:
4Z7A
Keywords:
Title:
Structural and biochemical characterization of a non-functionally redundant M. tuberculosis (3,3) L,D-Transpeptidase, LdtMt5.
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
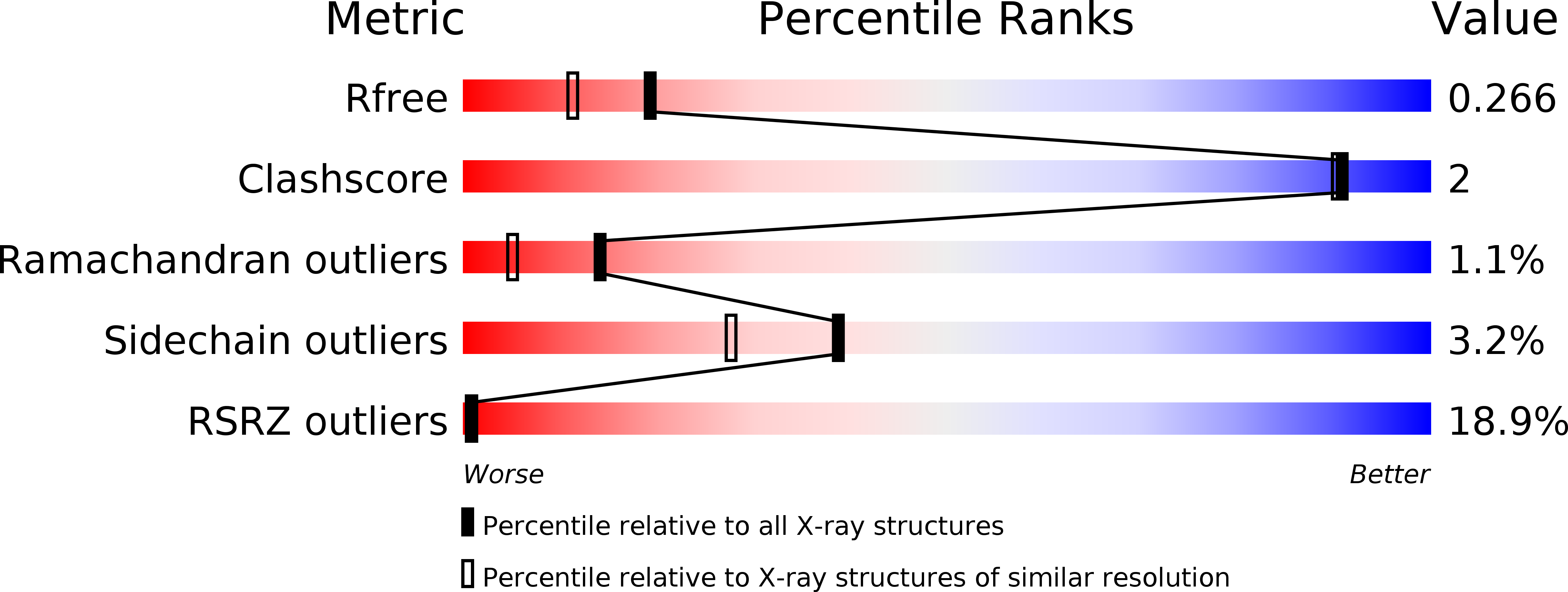
Resolution:
1.98 Å
R-Value Free:
0.26
R-Value Work:
0.21
R-Value Observed:
0.22
Space Group:
P 62 2 2