Deposition Date
2015-02-25
Release Date
2015-03-11
Last Version Date
2024-10-30
Entry Detail
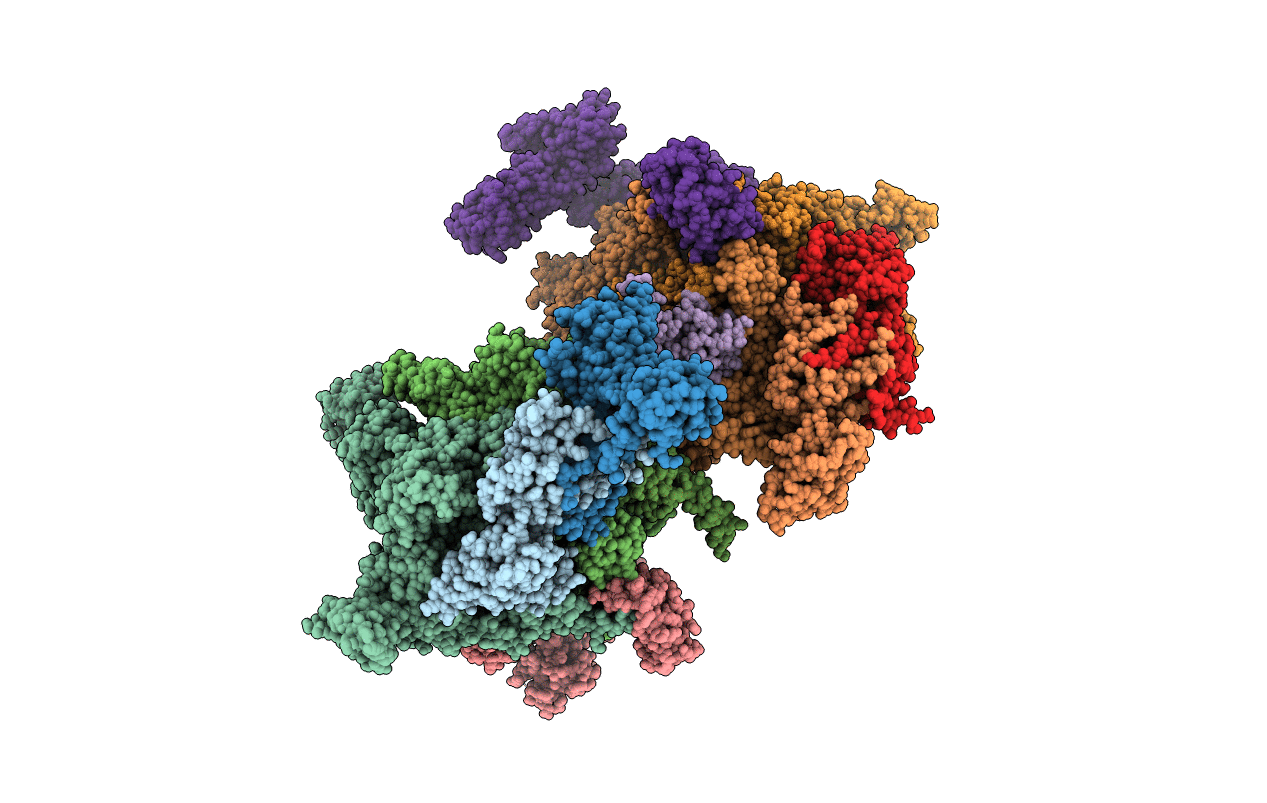
PDB ID:
4YFN
Keywords:
Title:
Escherichia coli RNA polymerase in complex with squaramide compound 14 (N-[3,4-dioxo-2-(4-{[4-(trifluoromethyl)benzyl]amino}piperidin-1-yl)cyclobut-1-en-1-yl]-3,5-dimethyl-1,2-oxazole-4-sulfonamide)
Biological Source:
Source Organism(s):
Escherichia coli O139:H28 (strain E24377A / ETEC) (Taxon ID: 331111)
Escherichia coli (strain K12) (Taxon ID: 83333)
Escherichia coli (strain K12) (Taxon ID: 83333)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.82 Å
R-Value Free:
0.26
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 21 21 21


