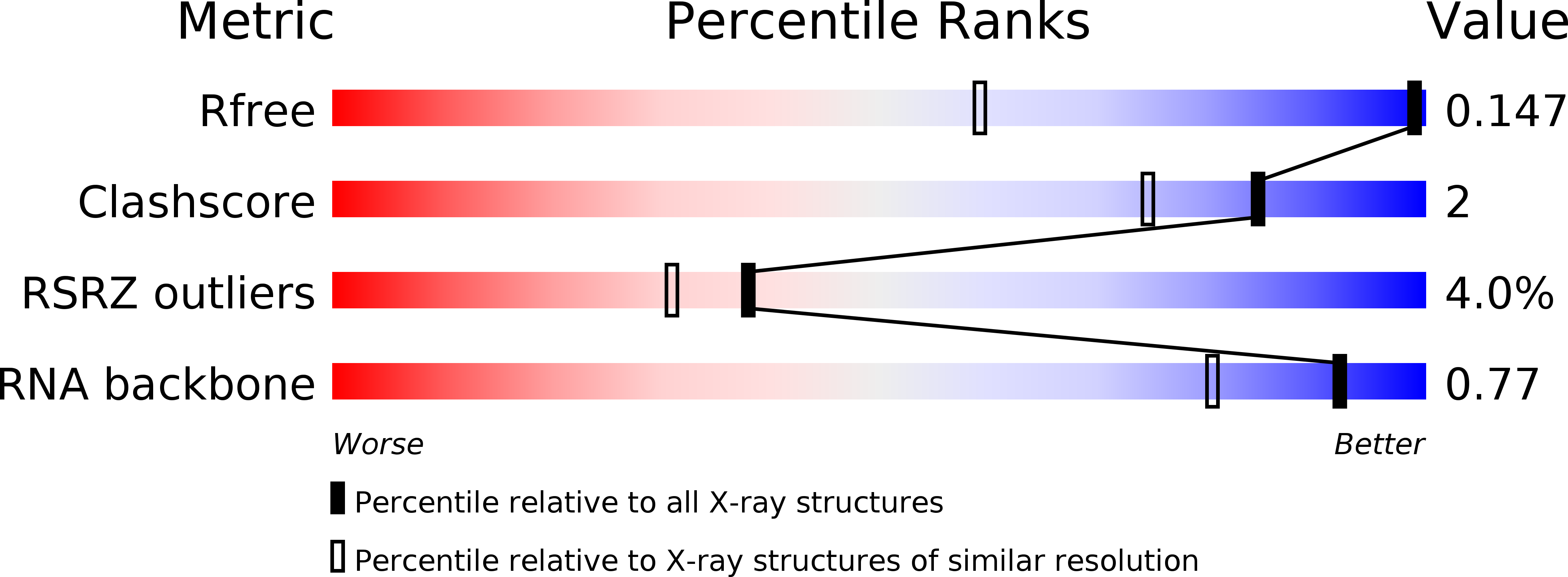
Deposition Date
2015-02-09
Release Date
2015-11-18
Last Version Date
2024-01-10
Entry Detail
PDB ID:
4Y27
Keywords:
Title:
E.coli 23S Sarcin-Ricil Loop, modified with a 2-Me on G2661 and a methylphosphonate on A2662
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
Method Details:
Experimental Method:
Resolution:
1.00 Å
R-Value Free:
0.14
R-Value Work:
0.13
R-Value Observed:
0.13
Space Group:
P 43