Deposition Date
2015-02-04
Release Date
2015-06-17
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4XZR
Keywords:
Title:
Structure of yeast importin a bound to the membrane protein Nuclear Localization Signal sequence of INM protein Heh1
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
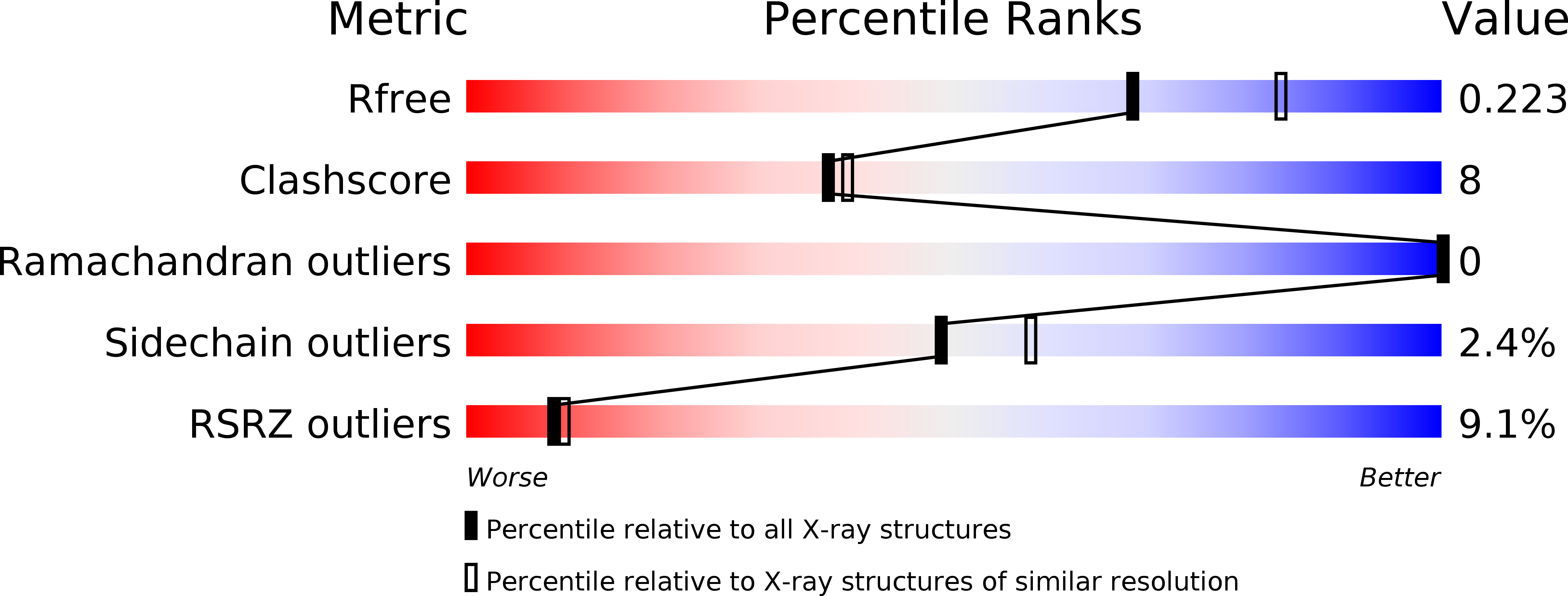
Resolution:
2.25 Å
R-Value Free:
0.21
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 1 2 1