Deposition Date
2015-02-03
Release Date
2016-01-27
Last Version Date
2024-11-13
Entry Detail
PDB ID:
4XZ3
Keywords:
Title:
Ca. Korarchaeum cryptofilum dinucleotide forming Acetyl-coenzyme A synthetase 1 (Se-Met derivative) in complex with coenzyme A and Mg-AMPPCP, phosphohistidine segment pointing towards nucleotide binding site
Biological Source:
Source Organism(s):
Candidatus Korarchaeum cryptofilum OPF8 (Taxon ID: 374847)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.40 Å
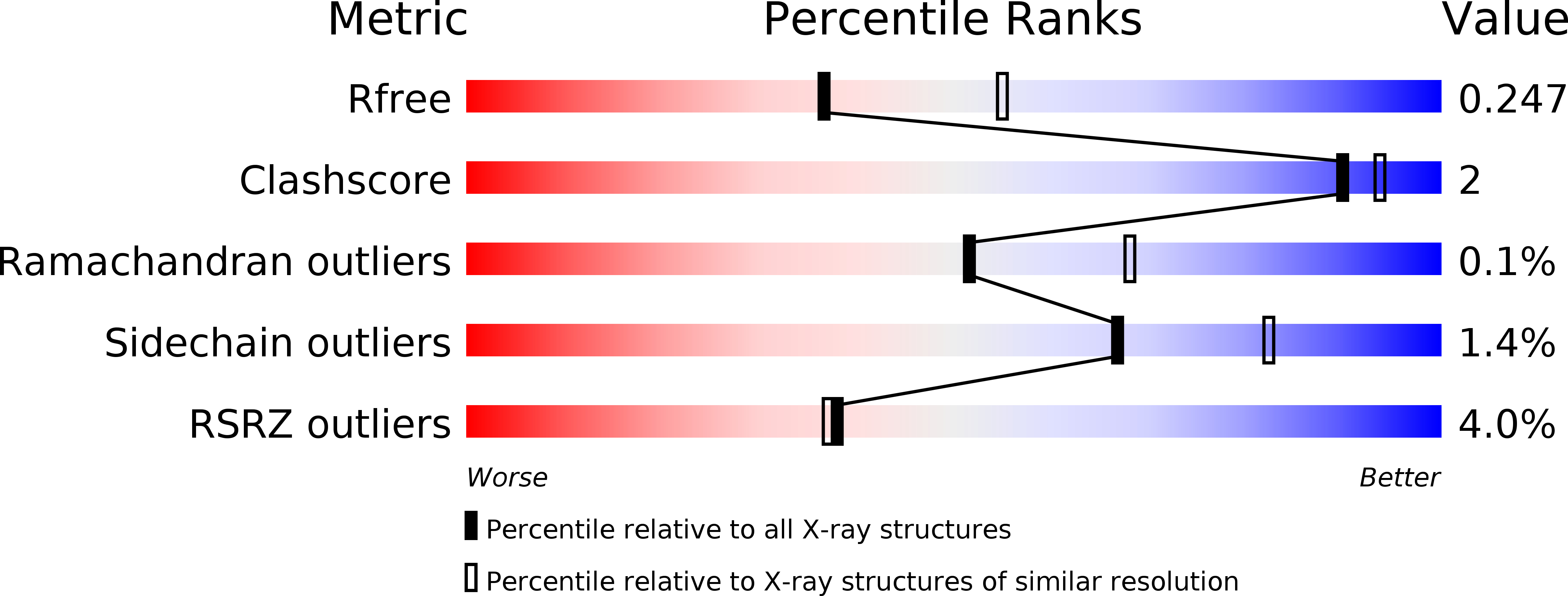
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21