Deposition Date
2015-01-26
Release Date
2016-01-27
Last Version Date
2025-08-20
Entry Detail
PDB ID:
4XUJ
Keywords:
Title:
Nucleosome core particle containing adducts from treatment with a thiomorpholine-substituted [(eta-6-p-cymene)Ru(3-hydroxy-2-pyridone)Cl] compound
Biological Source:
Source Organism(s):
Xenopus laevis (Taxon ID: 8355)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
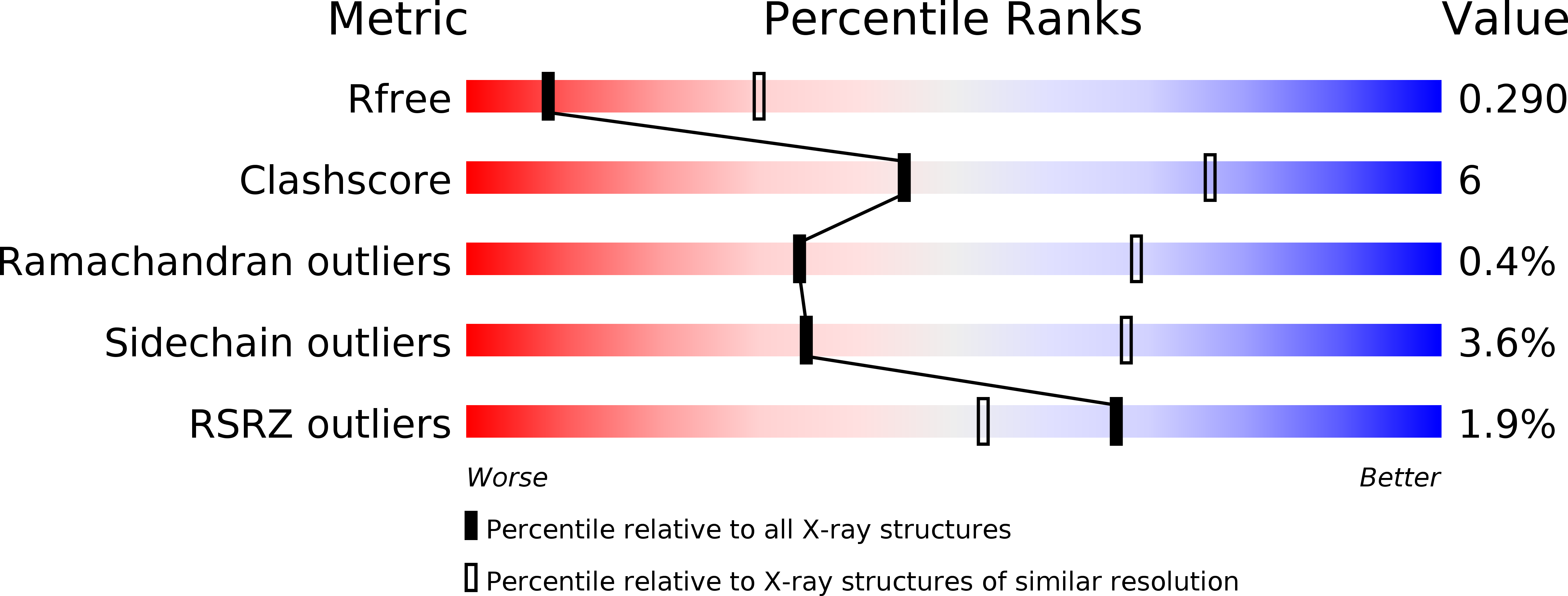
Resolution:
3.18 Å
R-Value Free:
0.29
R-Value Work:
0.26
R-Value Observed:
0.26
Space Group:
P 21 21 21