Deposition Date
2015-01-22
Release Date
2015-12-16
Last Version Date
2024-01-10
Entry Detail
PDB ID:
4XSD
Keywords:
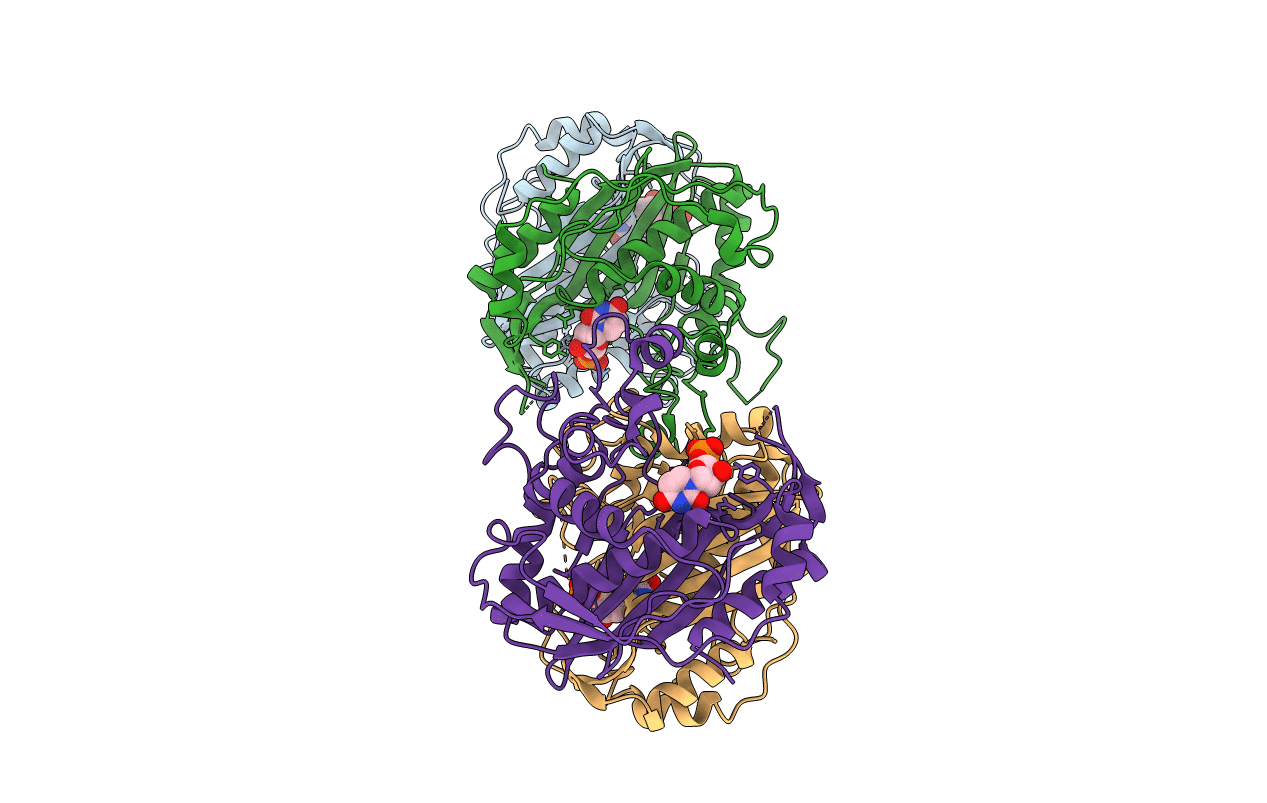
Title:
Complex structure of thymidylate synthase from varicella zoster virus with a dUMP
Biological Source:
Source Organism(s):
Varicella-zoster virus (strain Oka vaccine) (Taxon ID: 341980)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.90 Å
R-Value Free:
0.28
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
P 32


