Deposition Date
2015-01-16
Release Date
2015-06-10
Last Version Date
2024-01-10
Entry Detail
PDB ID:
4XOU
Keywords:
Title:
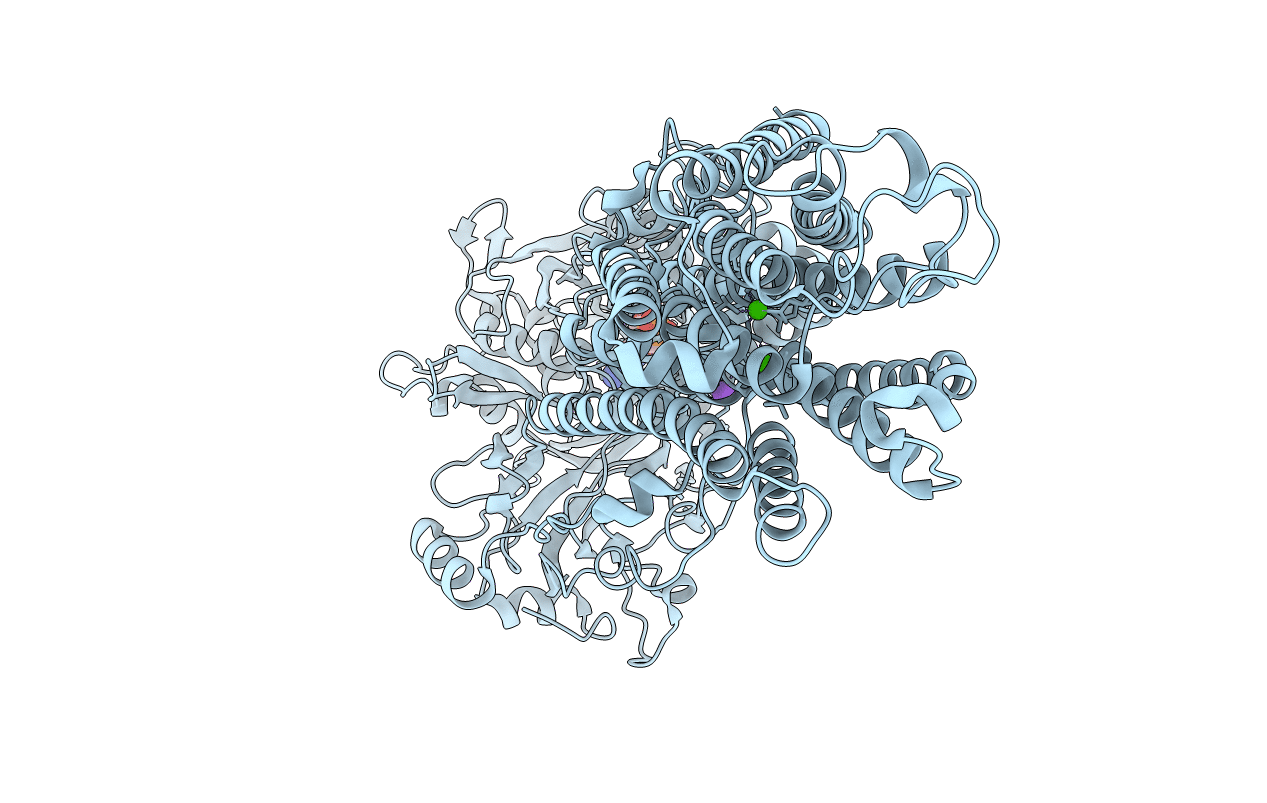
Crystal structure of the SR Ca2+-ATPase in the Ca2-E1-MgAMPPCP form determined by serial femtosecond crystallography using an X-ray free-electron laser.
Biological Source:
Source Organism(s):
Oryctolagus cuniculus (Taxon ID: 9986)
Method Details:
Experimental Method:
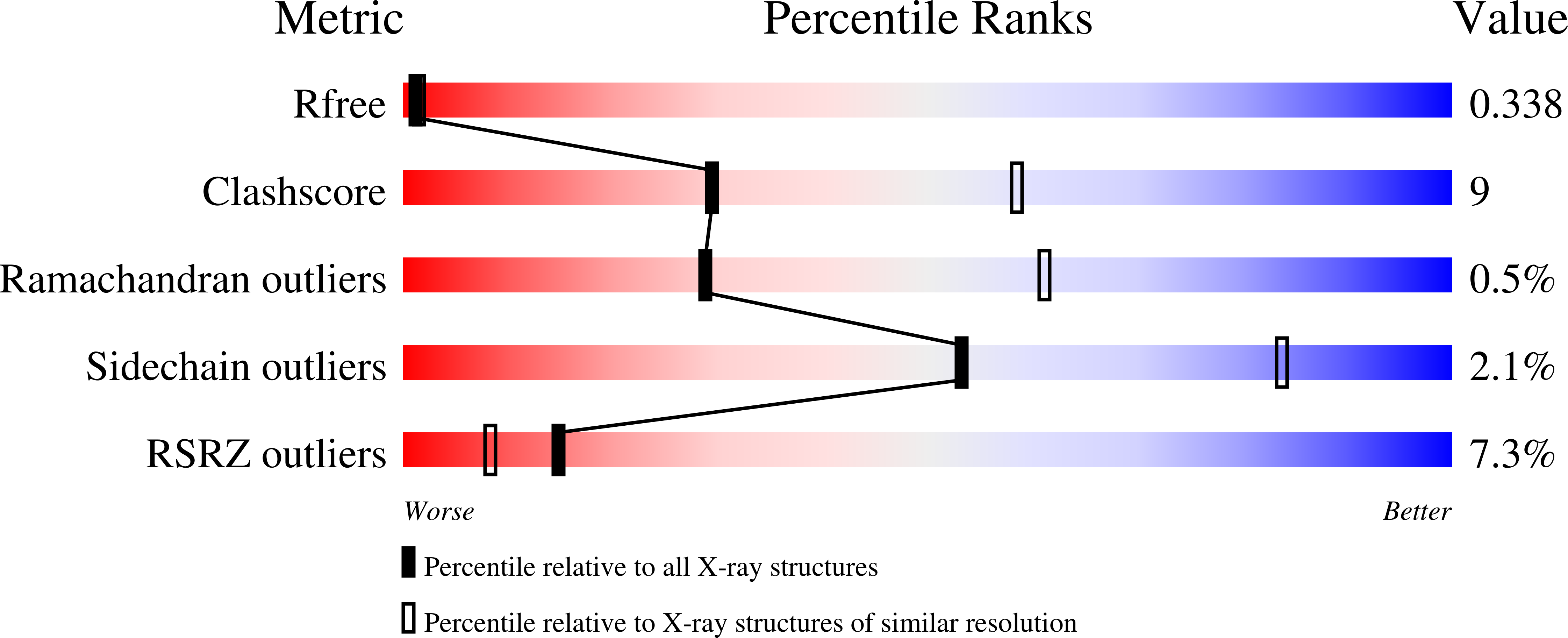
Resolution:
2.80 Å
R-Value Free:
0.34
R-Value Work:
0.30
R-Value Observed:
0.30
Space Group:
C 1 2 1