Deposition Date
2014-11-03
Release Date
2014-11-12
Last Version Date
2024-01-10
Entry Detail
PDB ID:
4WUO
Keywords:
Title:
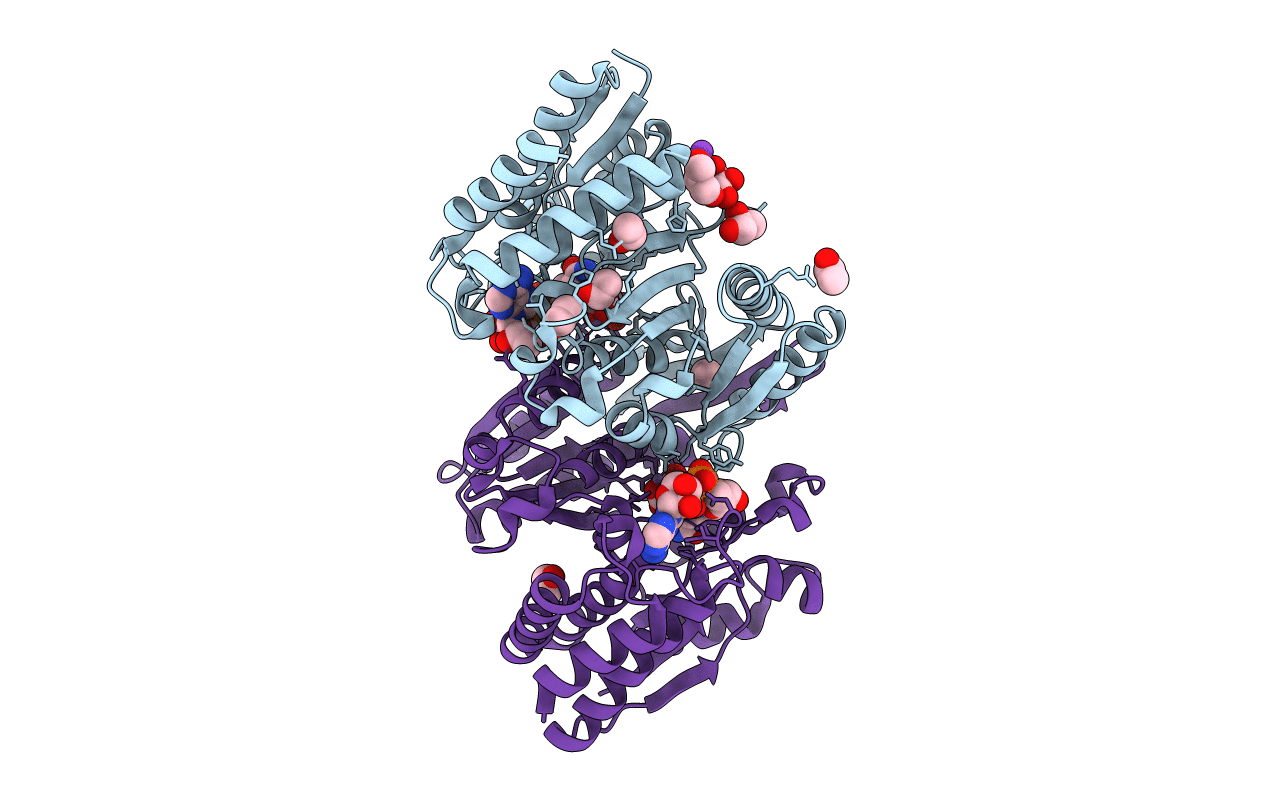
Structure of the E270A Mutant Isopropylmalate dehydrogenase from Thermus thermophilus in complex with IPM, Mn and NADH
Biological Source:
Source Organism:
Thermus thermophilus (Taxon ID: 300852)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.05 Å
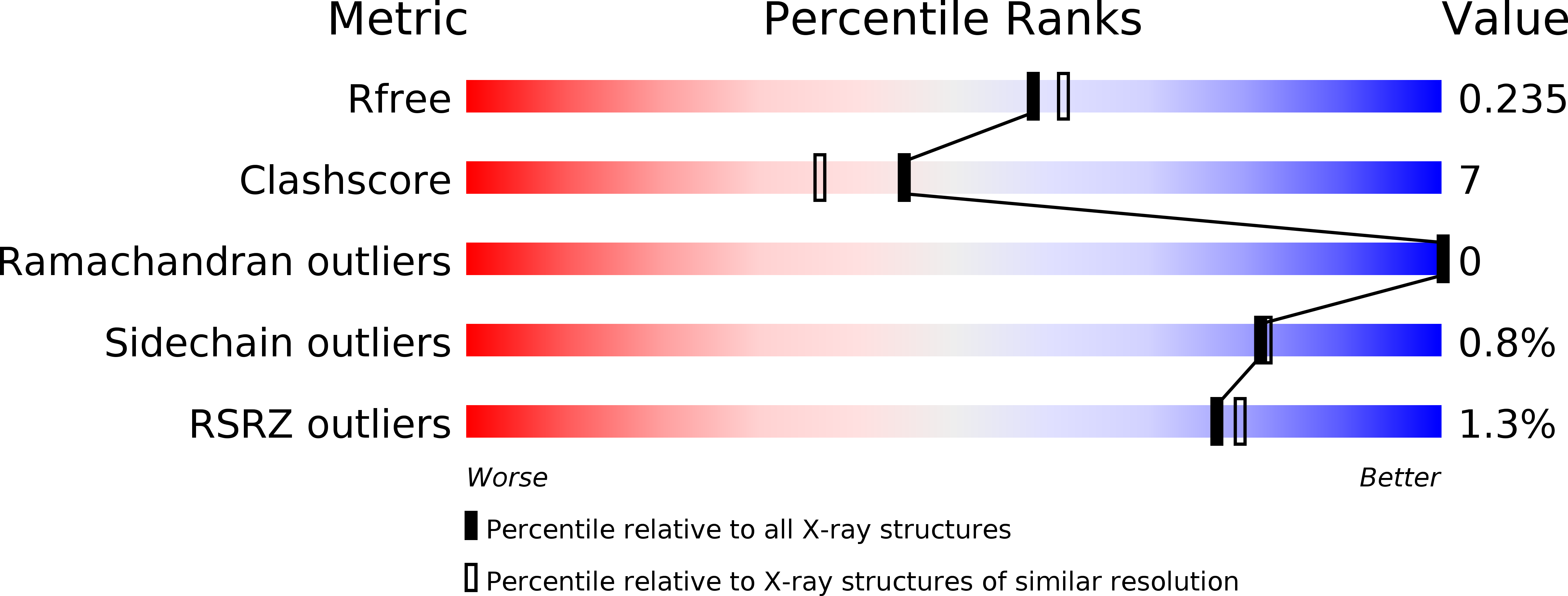
R-Value Free:
0.22
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 2 2 21