Deposition Date
2014-08-22
Release Date
2015-03-11
Last Version Date
2024-01-10
Entry Detail
PDB ID:
4W7K
Keywords:
Title:
CRYSTAL STRUCTURE OF A DECOLORIZING PEROXIDASE (DYP) FROM AURICULARIA AURICULA-JUDAE. Y147S MUTANT
Biological Source:
Source Organism(s):
Auricularia auricula-judae (Taxon ID: 29892)
Expression System(s):
Method Details:
Experimental Method:
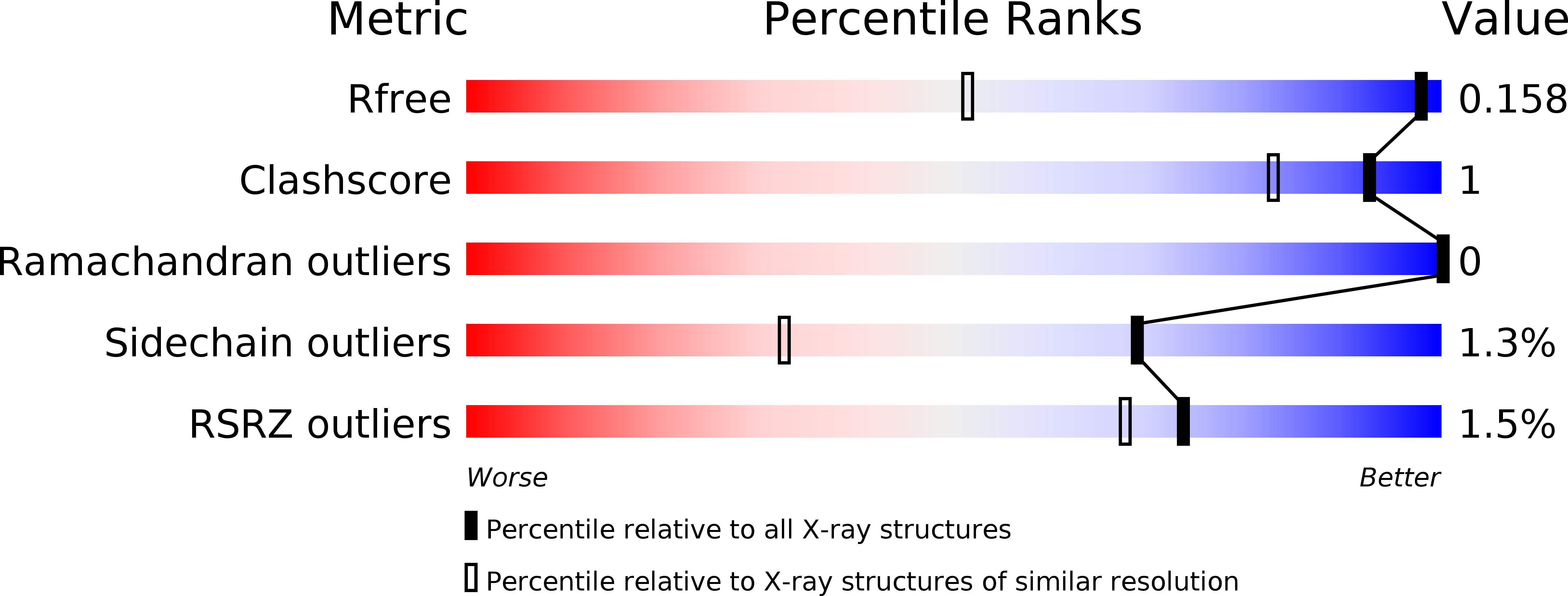
Resolution:
1.05 Å
R-Value Free:
0.15
R-Value Work:
0.13
R-Value Observed:
0.13
Space Group:
C 1 2 1