Deposition Date
2012-01-11
Release Date
2014-07-09
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4V94
Keywords:
Title:
Molecular architecture of the eukaryotic chaperonin TRiC/CCT derived by a combination of chemical crosslinking and mass-spectrometry, XL-MS
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 559292)
Expression System(s):
Method Details:
Experimental Method:
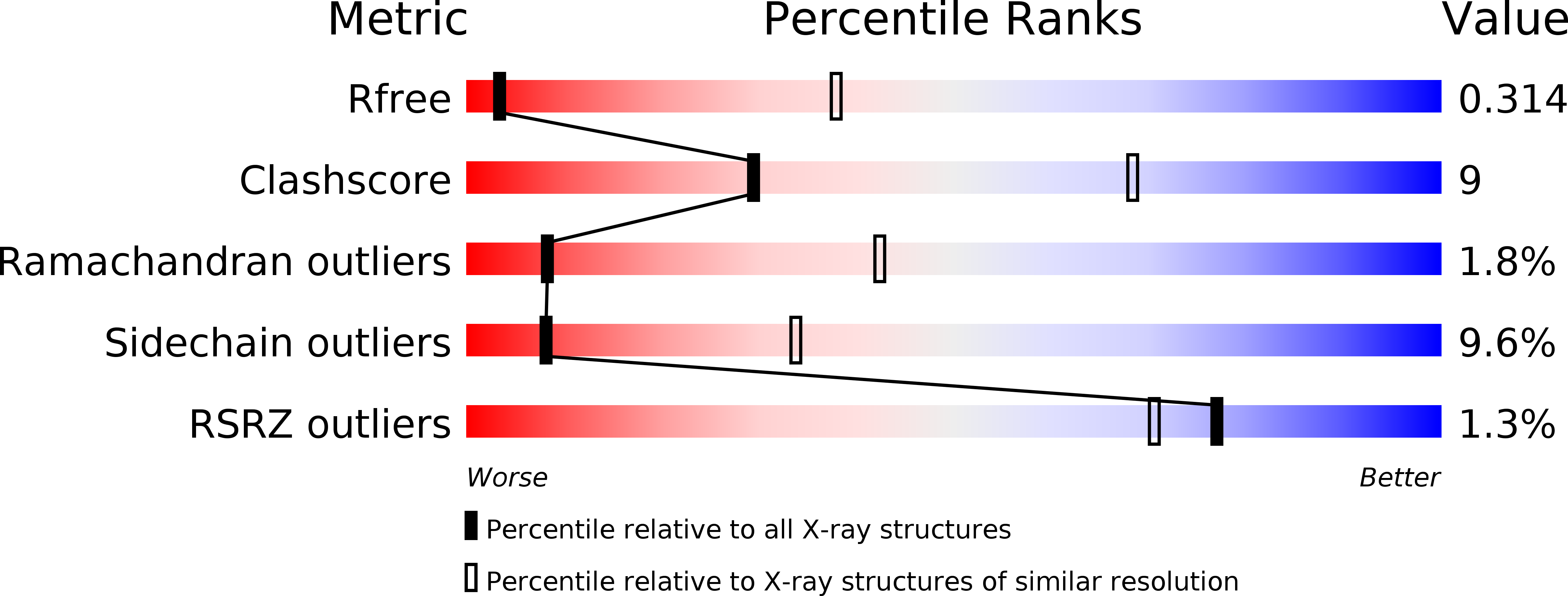
Resolution:
3.80 Å
R-Value Free:
0.30
R-Value Work:
0.25
R-Value Observed:
0.25
Space Group:
P 1