Deposition Date
2014-10-06
Release Date
2014-12-03
Last Version Date
2024-05-01
Entry Detail
PDB ID:
4V25
Keywords:
Title:
VER-246608, a novel pan-isoform ATP competitive inhibitor of pyruvate dehydrogenase kinase, disrupts Warburg metabolism and induces context- dependent cytostasis in cancer cells
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
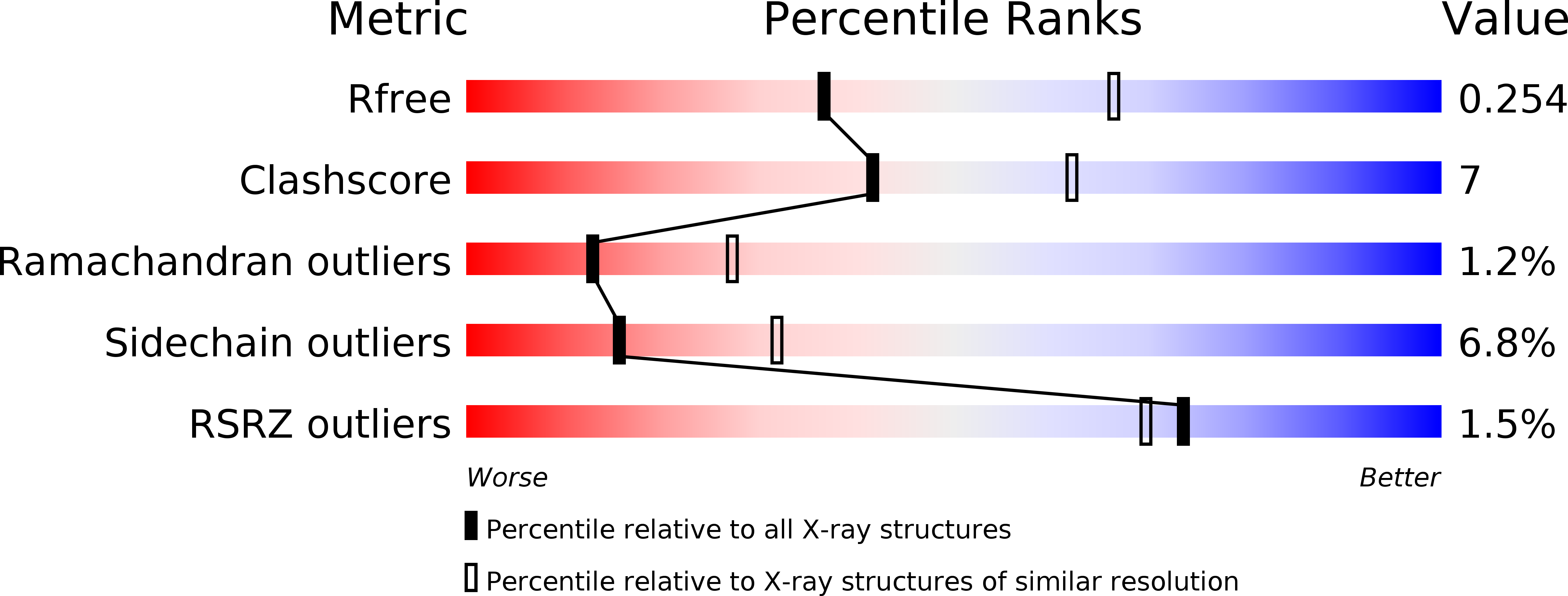
Resolution:
2.60 Å
R-Value Free:
0.25
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
P 64