Deposition Date
2014-09-05
Release Date
2015-05-13
Last Version Date
2024-01-10
Entry Detail
PDB ID:
4UZB
Keywords:
Title:
KSHV LANA (ORF73) C-terminal domain mutant bound to LBS1 DNA (R1039Q, R1040Q, K1055E, K1109A, D1110A, A1121E, K1138S, K1140D, K1141D)
Biological Source:
Source Organism(s):
HUMAN HERPESVIRUS 8 (Taxon ID: 37296)
Expression System(s):
Method Details:
Experimental Method:
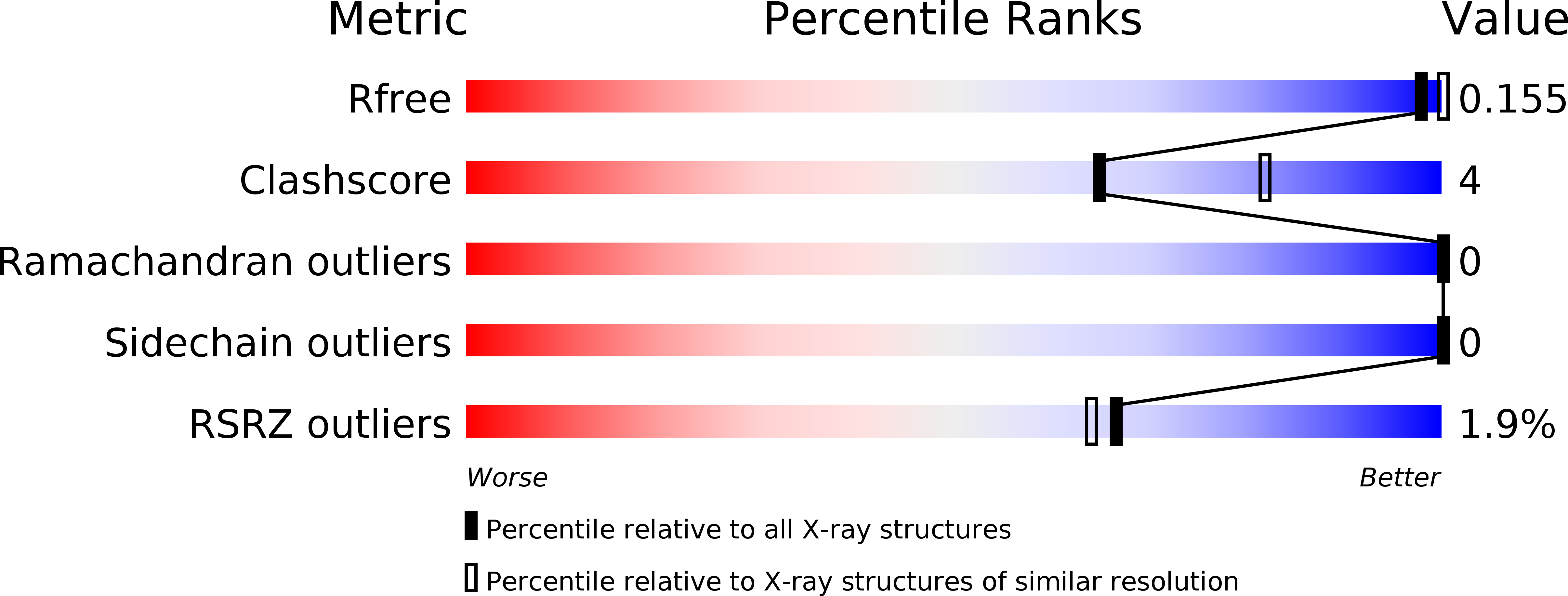
Resolution:
2.87 Å
R-Value Free:
0.15
R-Value Work:
0.12
R-Value Observed:
0.12
Space Group:
I 21 3