Deposition Date
2014-09-02
Release Date
2015-04-15
Last Version Date
2024-01-10
Entry Detail
PDB ID:
4UYP
Keywords:
Title:
High resolution structure of the third cohesin ScaC in complex with the ScaB dockerin with a mutation in the N-terminal helix (IN to SI) from Acetivibrio cellulolyticus displaying a type I interaction.
Biological Source:
Source Organism(s):
Acetivibrio cellulolyticus (Taxon ID: 35830)
Expression System(s):
Method Details:
Experimental Method:
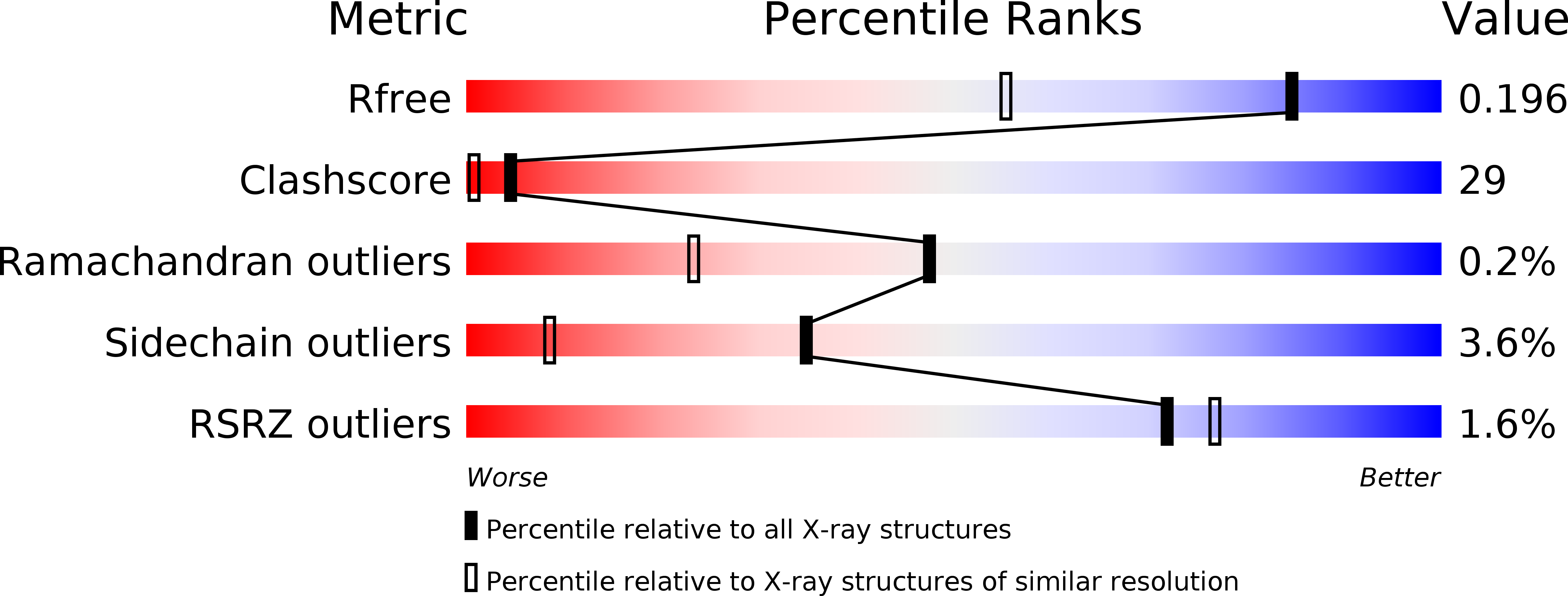
Resolution:
1.49 Å
R-Value Free:
0.18
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 41 21 2