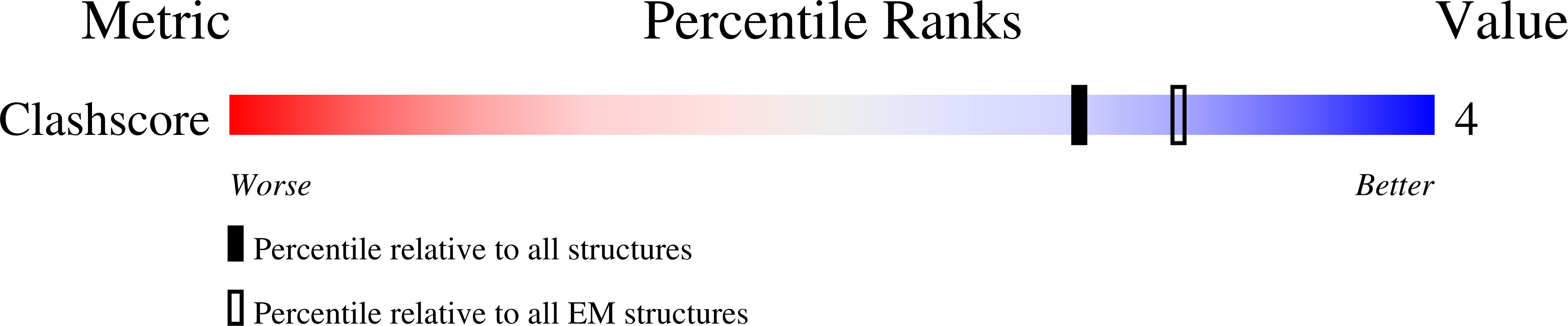Deposition Date
2014-06-15
Release Date
2014-08-20
Last Version Date
2024-05-08
Entry Detail
PDB ID:
4UPB
Keywords:
Title:
Electron cryo-microscopy of the complex formed between the hexameric ATPase RavA and the decameric inducible decarboxylase LdcI
Biological Source:
Source Organism(s):
ESCHERICHIA COLI K-12 (Taxon ID: 83333)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
11.00 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE