Deposition Date
2014-12-18
Release Date
2015-09-16
Last Version Date
2024-05-15
Entry Detail
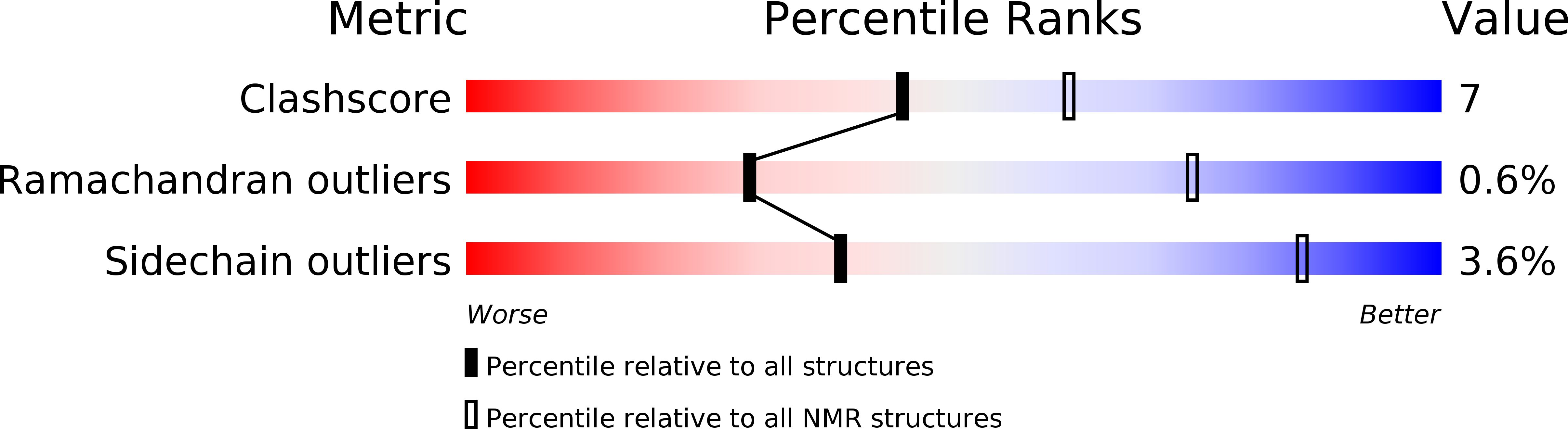
PDB ID:
4UET
Keywords:
Title:
Diversity in the structures and ligand binding sites among the fatty acid and retinol binding proteins of nematodes revealed by Na-FAR-1 from Necator americanus
Biological Source:
Source Organism(s):
NECATOR AMERICANUS (Taxon ID: 51031)
Expression System(s):
Method Details:
Experimental Method:
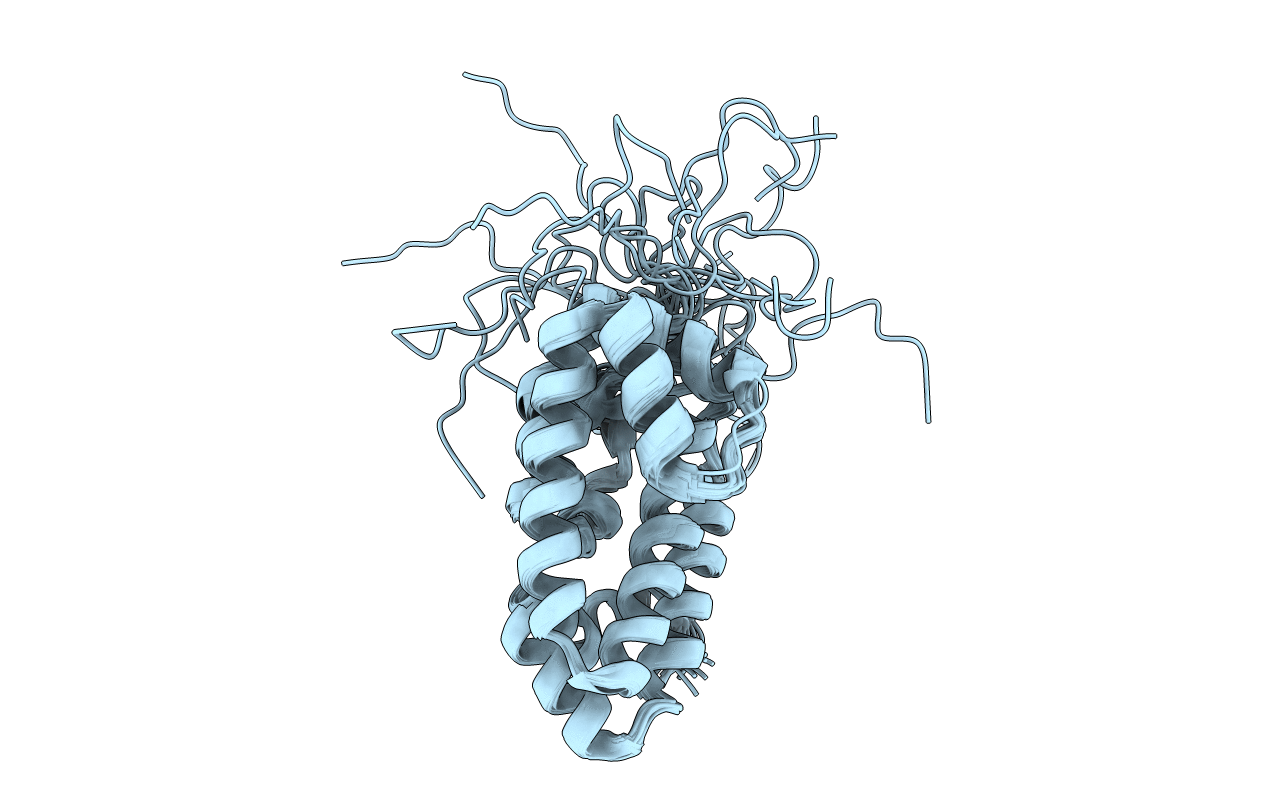
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
BEST RESTRAINT ENERGY