Deposition Date
2014-07-18
Release Date
2015-01-14
Last Version Date
2024-10-09
Entry Detail
PDB ID:
4U2W
Keywords:
Title:
Atomic resolution crystal structure of HV-BBI protease inhibitor from amphibian skin in complex with bovine trypsin
Biological Source:
Source Organism(s):
Odorrana versabilis (Taxon ID: 326940)
Bos taurus (Taxon ID: 9913)
Bos taurus (Taxon ID: 9913)
Method Details:
Experimental Method:
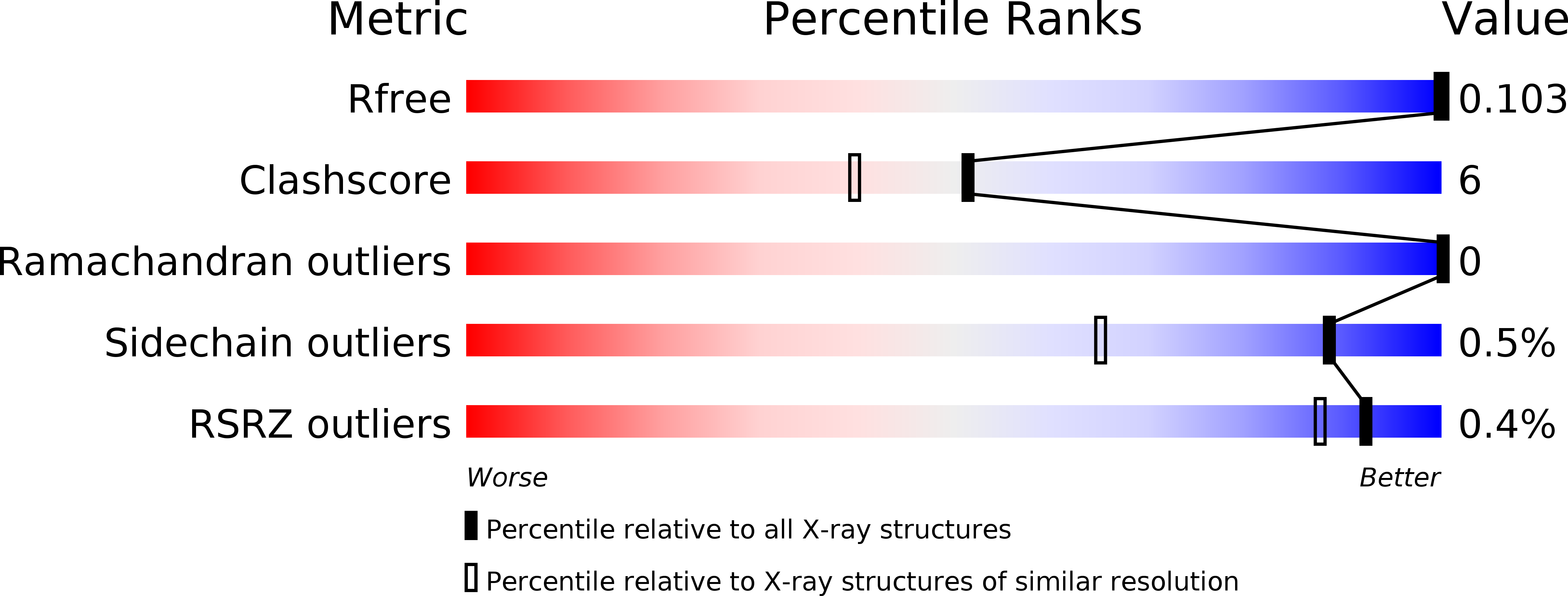
Resolution:
1.00 Å
R-Value Free:
0.10
R-Value Work:
0.09
R-Value Observed:
0.09
Space Group:
P 21 21 21