Deposition Date
2014-07-08
Release Date
2015-05-06
Last Version Date
2024-03-20
Entry Detail
PDB ID:
4TYA
Keywords:
Title:
An Ligand-observed Mass Spectrometry-based Approach Integrated into the Fragment Based Lead Discovery Pipeline
Biological Source:
Source Organism(s):
Hepatitis C virus (Taxon ID: 11103)
Expression System(s):
Method Details:
Experimental Method:
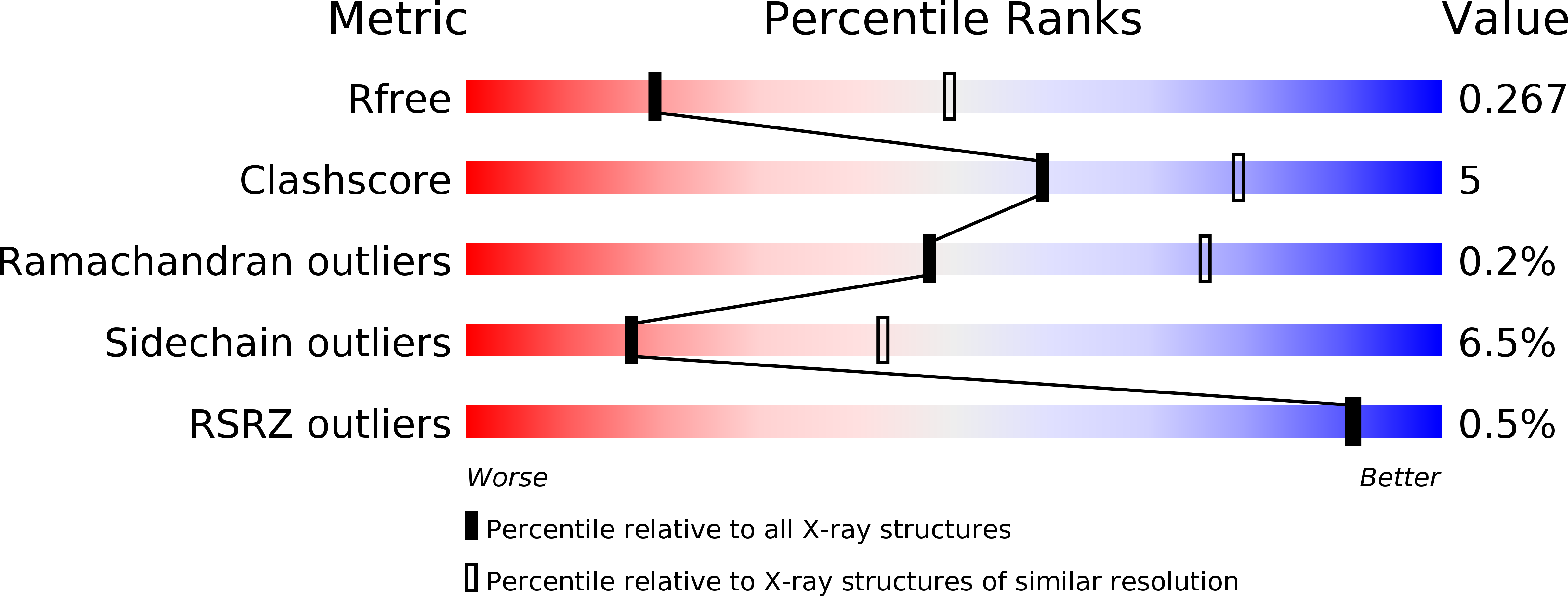
Resolution:
2.94 Å
R-Value Free:
0.27
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 21 21 21