Deposition Date
2014-08-03
Release Date
2015-04-29
Last Version Date
2024-10-16
Entry Detail
PDB ID:
4R10
Keywords:
Title:
A conserved phosphorylation switch controls the interaction between cadherin and beta-catenin in vitro and in vivo
Biological Source:
Source Organism(s):
Caenorhabditis elegans (Taxon ID: 6239)
Expression System(s):
Method Details:
Experimental Method:
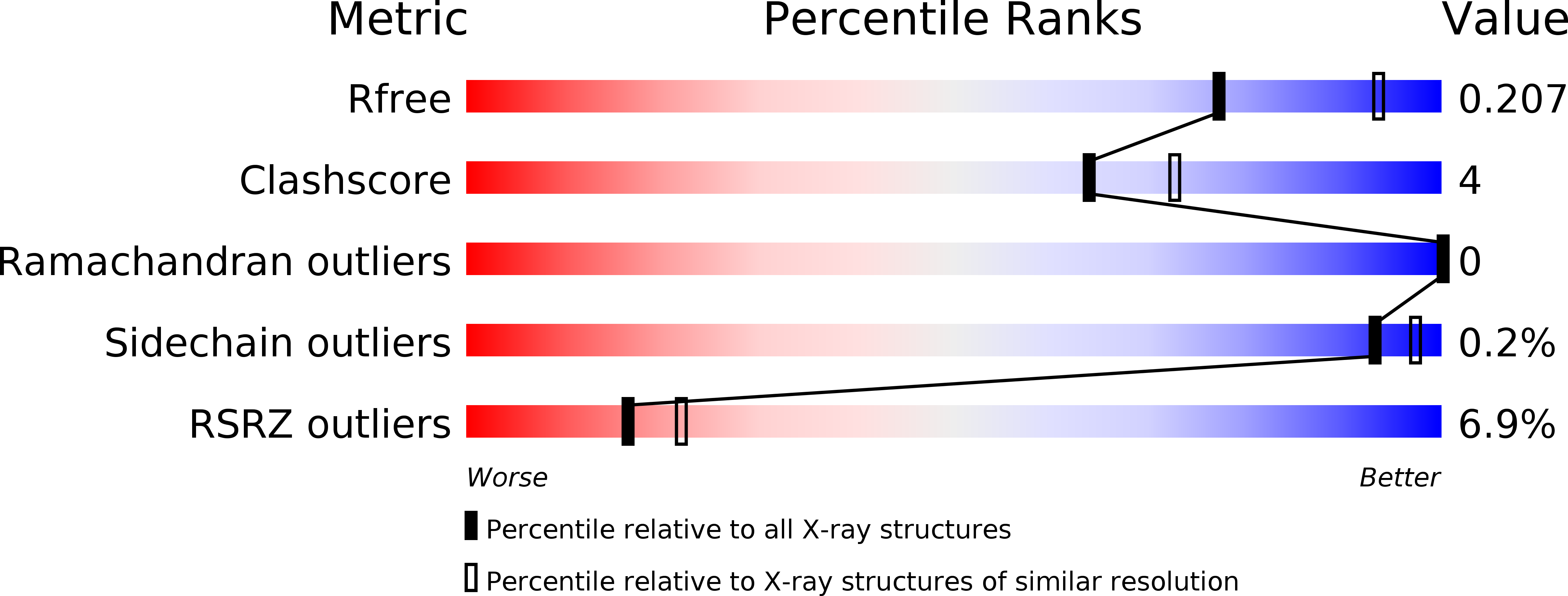
Resolution:
2.30 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 43